Suppressed ion migration in halide perovskite nanocrystals by simultaneous Ni2+ doping and halogen vacancy filling
-
摘要: 卤化铅钙钛矿(LHPs)由于具有优异的光电性能和制备成本低等优点,已成为新一代光电器件的有力候选材料。然而,缺陷造成的离子迁移会导致LHPs纳米晶的晶体结构解离分解。因此,稳定性成为LHPs实际应用中亟待解决的问题。本文旨在研究镍离子替位掺杂及卤素空位填补对CsPbBr3纳米晶中的离子迁移抑制作用。通过离子迁移活化能的测定和高分辨透射电镜的原位观察,分析了前驱体掺杂剂对加强LHPs稳定性的作用原理。首先,选用乙酰丙酮镍和溴化镍作为掺杂剂,合成了掺杂LHPs纳米晶。其次,通过吸收-荧光光谱,X射线衍射,X射线光电子衍射,透射电子显微镜等测试手段对掺杂样品的光学及化学组成进行分析。最后,通过纳米晶薄膜电导率的温度依赖关系计算出其离子迁移活化能,并结合高分辨电镜原位观察纳米晶在高能电子束辐照下的形貌演变过程,揭示了不同掺杂剂对合成掺杂LHPs稳定性的影响。实验结果表明:Ni2+掺杂CsPbBr3样品的离子迁移活化能相较本征CsPbBr3样品(0.07 eV)有显著提升,其中乙酰丙酮镍掺杂样品的离子迁移活化能为0.238 eV,溴化镍掺杂样品的离子迁移活化能为0.487 eV。另外,电子束辐照测试表明溴化镍掺杂钙钛矿晶体表现出更高的结构稳定性,这主要归因于掺杂的Ni2+对卤素的强结合和卤素填补空位缺陷的协同钝化作用。Ni2+掺杂和卤素空位填充协同可以有效抑制卤化物钙钛矿纳米晶体中的离子迁移。Abstract: Lead Halide Perovskites(LHPs) are promising candidates for next-generation optoelectronic application. However, defect-induced ion migration causes phase degradation in LHP nanocrystals. Therefore, material stability has become an urgent problem that impedes practical applications. In this paper, we aim to study the influence of doping cations on inhibiting the migration of halogen ions in perovskite nanocrystals. Through the measurement of ion migration activation energy and in-situ high-resolution transmission electron microscope technology, the effect of precursor dopants on the stability of LHPs were analyzed. Firstly, we synthesized two types of LHP nanocrystals with high crystal quality using nickel acetylacetonate and nickel bromide as precursor dopants, respectively. Secondly, the optical properties and component elements of the doped samples were analyzed by absorption-fluorescence spectroscopy, X-ray diffraction, X-ray photoelectron diffraction, and transmission electron microscopy. Finally, the ion migration activation energies of various LHP films were measured using temperature-dependent ion conductivity tests, and the influence of the precursor dopants on the stability of as-synthesized doped LHPs was compared with the results from high-resolution electron microscopy. The results showed that the activation energies of the doped CsPbBr3 samples were significantly improved compared to the intrinsic CsPbBr3 sample (0.07 eV), which were determined to be 0.238 eV for nickel acetylacetonate and 0.487 eV for nickel bromide. In addition, the electron irradiation experiments showed that the nickel bromide-doped perovskite nanocrystals exhibited higher structural stability. This is due to the strong bonding of doped Ni2+ to halogen and the synergistic passivation effect of halogen filling vacancy defects. It can be concluded that Ni2+ doping and halogen vacancy filling can effectively inhibit ion migration in halide perovskite nanocrystals.
-
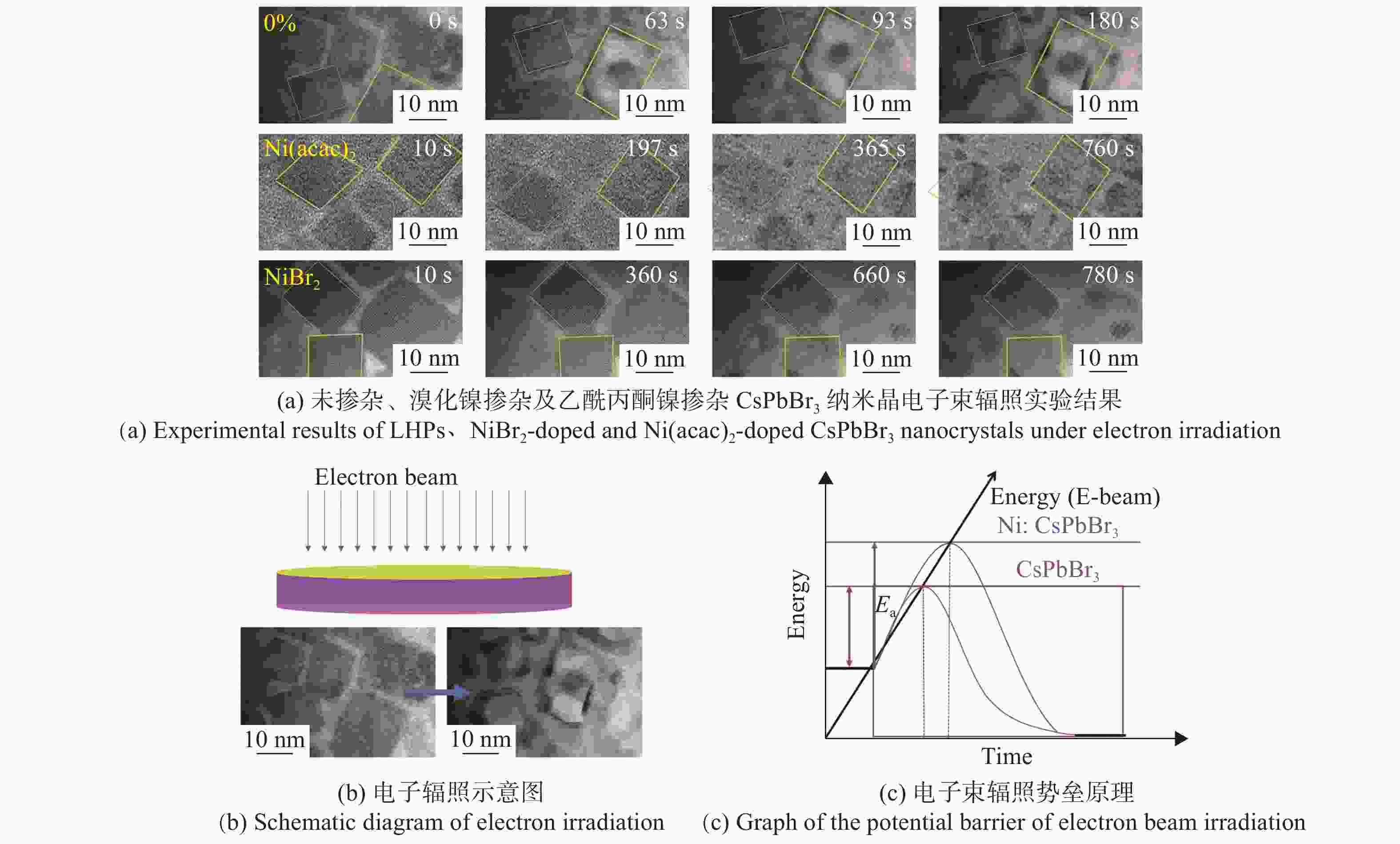
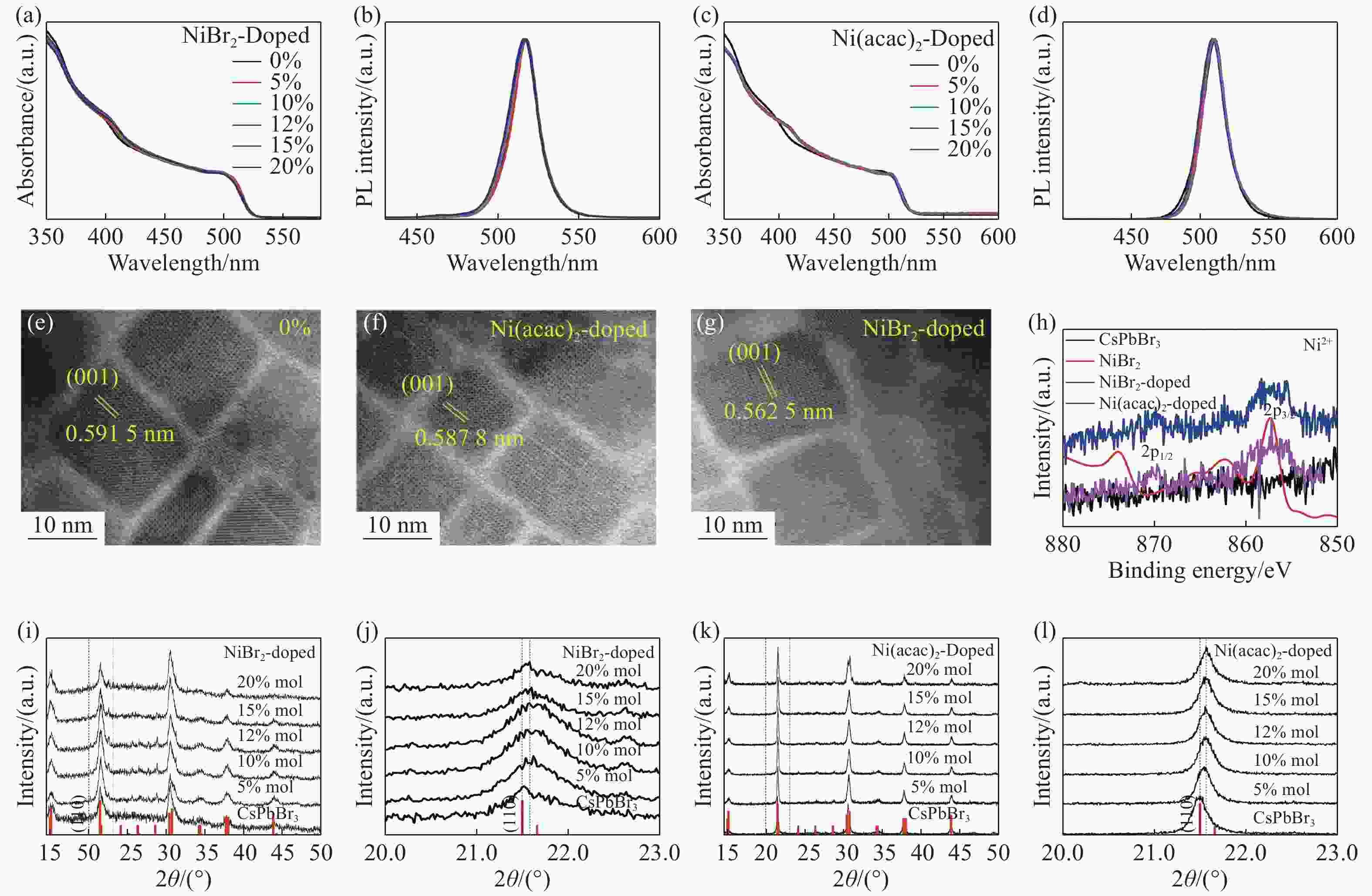
图 1 钙钛矿样品的结构和性能。(a, b)为溴化镍掺杂(NiBr2-doped)CsPbBr3纳米晶的吸收光谱和发射光谱。(c, d) 乙酰丙酮镍掺杂(Ni(acac)2-doped))CsPbBr3纳米晶吸收光谱和发射光谱。(e, f, g)分别为未掺杂,乙酰丙酮镍掺杂及溴化镍掺杂CsPbBr3纳米晶体TEM图像。(h) 掺Ni的CsPbBr3纳米晶与本征CsPbBr3的XPS图谱。 (i,k)分别为NiBr2-doped、Ni(acac)2-doped CsPbBr3纳米晶的XRD图谱,在图的底部显示了斜方CsPbBr3结构。(j, l)分别为Ni(acac)2-doped、NiBr2-doped CsPbBr3纳米晶的高分辨XRD谱(110)(JCPDS No.18-0364)
Figure 1. Structures and properties of LHPs. Absorption spectra and PL spectra of CsPbBr3 nanocrystals doped with NiBr2 (a, b) and Ni(acac)2 (c,d). (e, f, g) TEM images of Ni-doped and undoped CsPbBr3 nanocrystals. (h) XPS spectra of Ni-doped and undoped CsPbBr3 nanocrystals. (i, k) XRD patterns of NiBr2-doped, Ni(acac)2-doped and undoped CsPbBr3 nanocrystals. JCPDS No.18-0364 of the orthorhombic CsPbBr3 structure are indicated at the bottom of each figure. (j, l) High-resolution XRD spectra (110) of NiBr2-doped, Ni(acac)2-doped and undoped CsPbBr3 nanocrystals
图 3 活化能测试示意图及测试结果。(a)活化能测试示意图,图中t1和t2指光电流和暗电流分别拟合得到的光/暗电流的驰豫时间。(b)不同温度暗电流变化(NiBr2掺杂10%),插图显示以电流弛豫时间为横坐标的半对数暗电流曲线(lnA)。(c)阿伦尼乌斯关系式活化能(Ea)计算结果。(d)不同掺杂样品的活化能 (x代表实际含量)
Figure 3. Schematic diagram of activation energy test and test results. (a) Schematic diagram of activation energy test. t1 and t2 in the figure refer to the relaxation time of the light/dark currents fitted by the photocurrent and dark current, respectively. (b) The dark current changes at different temperatures (NiBr2 doping 10%). The inset shows the semilog dark current (lnA) curve as function with the current relaxation time. (c) Activation energy (Ea) caculated by formla (1). (d) Activation energy of different doped samples (x is the actual content)
表 1 使用ICP-MS测试的掺杂含量
Table 1. Doping content tested by ICP-MS
乙酰丙酮镍掺杂 溴化镍掺杂 名义含量 实际含量* 名义含量 实际含量 5% 0.07 5% 0.11 10% 0.12 10% 0.14 12% 0.13 12% 0.17 15% 0.20 15% 0.21 20% 0.23 20% 0.25 *:表示ICP-MS测定的CsPb1−xNixBr3中Ni掺杂的实际含量 -
[1] KOJIMA A, TESHIMA K, SHIRAI Y, et al. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells[J]. Journal of the American Chemical Society, 2009, 131(17): 6050-6051. doi: 10.1021/ja809598r [2] YANG W S, NOH J H, JEON N J, et al. High-performance photovoltaic perovskite layers fabricated through intramolecular exchange[J]. Science, 2015, 348(6240): 1234-1237. doi: 10.1126/science.aaa9272 [3] SONG J ZH, LI J H, LI X M, et al. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3)[J]. Advanced Materials, 2015, 27(44): 7162-7167. doi: 10.1002/adma.201502567 [4] LI X, BI D Q, YI CH Y, et al. A vacuum flash-assisted solution process for high-efficiency large-area perovskite solar cells[J]. Science, 2016, 353(6294): 58-62. doi: 10.1126/science.aaf8060 [5] JEON N J, NA H, JUNG E H, et al. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells[J]. Nature Energy, 2018, 3(8): 682-689. doi: 10.1038/s41560-018-0200-6 [6] JUNG E H, JEON N J, PARK E Y, et al. Efficient, stable and scalable perovskite solar cells using poly(3-hexylthiophene)[J]. Nature, 2019, 567(7749): 511-515. doi: 10.1038/s41586-019-1036-3 [7] MOMBLONA C, GIL-ESCRIG L, BANDIELLO E, et al. Efficient vacuum deposited p-i-n and n-i-p perovskite solar cells employing doped charge transport layers[J]. Energy &Environmental Science, 2016, 9(11): 3456-3463. [8] TAN H R, JAIN A, VOZNYY O, et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation[J]. Science, 2017, 355(6326): 722-726. doi: 10.1126/science.aai9081 [9] TRESS W, MARINOVA N, MOEHL T, et al. Understanding the rate-dependent J-V hysteresis, slow time component, and aging in CH3NH3PbI3 perovskite solar cells: the role of a compensated electric field[J]. Energy &Environmental Science, 2015, 8(3): 995-1004. [10] MCMEEKIN D P, SADOUGHI G, REHMAN W, et al. A mixed-cation lead mixed-halide perovskite absorber for tandem solar cells[J]. Science, 2016, 351(6269): 151-155. doi: 10.1126/science.aad5845 [11] CHEN M M, SHAN X, GESKE T, et al. Manipulating ion migration for highly stable light-emitting diodes with single-crystalline organometal halide perovskite microplatelets[J]. ACS Nano, 2017, 11(6): 6312-6318. doi: 10.1021/acsnano.7b02629 [12] DEQUILETTES D W, ZHANG W, BURLAKOV V M, et al. Photo-induced halide redistribution in organic-inorganic perovskite films[J]. Nature Communications, 2016, 7: 11683. doi: 10.1038/ncomms11683 [13] EAMES C, FROST J M, BARNES P R F, et al. Ionic transport in hybrid lead iodide perovskite solar cells[J]. Nature Communications, 2015, 6: 7497. doi: 10.1038/ncomms8497 [14] LI CH, GUERRERO A, HUETTNER S, et al. Unravelling the role of vacancies in lead halide perovskite through electrical switching of photoluminescence[J]. Nature Communications, 2018, 9(1): 5113. doi: 10.1038/s41467-018-07571-6 [15] LAI M L, OBLIGER A, LU D, et al. Intrinsic anion diffusivity in lead halide perovskites is facilitated by a soft lattice[J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(47): 11929-11934. doi: 10.1073/pnas.1812718115 [16] SHAO Y CH, FANG Y J, LI T, et al. Grain boundary dominated ion migration in polycrystalline organic-inorganic halide perovskite films[J]. Energy &Environmental Science, 2016, 9(5): 1752-1759. [17] YUN J S, SEIDEL J, KIM J, et al. Critical role of grain boundaries for ion migration in formamidinium and methylammonium lead halide perovskite solar cells[J]. Advanced Energy Materials, 2016, 6(13): 1600330. doi: 10.1002/aenm.201600330 [18] YUAN Y B, CHAE J, SHAO Y CH, et al. Photovoltaic switching mechanism in lateral structure hybrid perovskite solar cells[J]. Advanced Energy Materials, 2015, 5(15): 1500615. doi: 10.1002/aenm.201500615 [19] VASHISHTHA P, HALPERT J E. Field-driven ion migration and color instability in red-emitting mixed halide perovskite nanocrystal light-emitting diodes[J]. Chemistry of Materials, 2017, 29(14): 5965-5973. doi: 10.1021/acs.chemmater.7b01609 [20] ZHANG H CH, FU X, TANG Y, et al. Phase segregation due to ion migration in all-inorganic mixed-halide perovskite nanocrystals[J]. Nature Communications, 2019, 10(1): 1088. doi: 10.1038/s41467-019-09047-7 [21] CAO J, TAO SH X, BOBBERT P A, et al. Interstitial occupancy by extrinsic alkali cations in perovskites and its impact on ion migration[J]. Advanced Materials, 2018, 30(26): 1707350. doi: 10.1002/adma.201707350 [22] XING J, WANG Q, DONG Q F, et al. Ultrafast ion migration in hybrid perovskite polycrystalline thin films under light and suppression in single crystals[J]. Physical Chemistry Chemical Physics, 2016, 18(44): 30484-30490. doi: 10.1039/C6CP06496E [23] CHEN SH L, ZHANG X W, ZHAO J J, et al. Atomic scale insights into structure instability and decomposition pathway of methylammonium lead iodide perovskite[J]. Nature Communications, 2018, 9(1): 4807. doi: 10.1038/s41467-018-07177-y [24] WEI D, MA F SH, WANG R, et al. Ion-migration inhibition by the cation-π interaction in perovskite materials for efficient and stable perovskite solar cells[J]. Advanced Materials, 2018, 30(31): 1707583. doi: 10.1002/adma.201707583 [25] ZHANG X Y, LU M, ZHANG Y, et al. PbS capped CsPbI3 nanocrystals for efficient and stable light-emitting devices using p-i-n structures[J]. ACS Central Science, 2018, 4(10): 1352-1359. doi: 10.1021/acscentsci.8b00386 [26] WANG CH J, CHESMAN A S R, JASIENIAK J J. Stabilizing the cubic perovskite phase of CsPbI3 nanocrystals by using an alkyl phosphinic acid[J]. Chemical Communications, 2017, 53(1): 232-235. doi: 10.1039/C6CC08282C [27] LIN Y, BAI Y, FANG Y J, et al. Suppressed ion migration in low-dimensional perovskites[J]. ACS Energy Letters, 2017, 2(7): 1571-1572. doi: 10.1021/acsenergylett.7b00442 [28] WANG X, LING Y CH, LIAN X J, et al. Suppressed phase separation of mixed-halide perovskites confined in endotaxial matrices[J]. Nature Communications, 2019, 10(1): 695. doi: 10.1038/s41467-019-08610-6 [29] XING J, ZHAO Y B, ASKERKA M, et al. Color-stable highly luminescent sky-blue perovskite light-emitting diodes[J]. Nature Communications, 2018, 9(1): 3541. doi: 10.1038/s41467-018-05909-8 [30] ZOU SH H, LIU Y SH, LI J H, et al. Stabilizing cesium lead halide perovskite lattice through MN(Ⅱ) substitution for air-stable light-emitting diodes[J]. Journal of the American Chemical Society, 2017, 139(33): 11443-11450. doi: 10.1021/jacs.7b04000 [31] PAROBEK D, ROMAN B J, DONG Y T, et al. Exciton-to-dopant energy transfer in Mn-doped cesium lead halide perovskite nanocrystals[J]. Nano Letters, 2016, 16(12): 7376-7380. doi: 10.1021/acs.nanolett.6b02772 [32] BI CH H, WANG SH X, LI Q, et al. Thermally stable copper(Ⅱ)-doped cesium lead halide perovskite quantum dots with strong blue emission[J]. The Journal of Physical Chemistry Letters, 2019, 10(5): 943-952. doi: 10.1021/acs.jpclett.9b00290 [33] YUAN X, JI S H, DE SIENA M C, et al. Photoluminescence temperature dependence, dynamics, and quantum efficiencies in Mn2+-doped CsPbCl3 perovskite nanocrystals with varied dopant concentration[J]. Chemistry of Materials, 2017, 29(18): 8003-8011. doi: 10.1021/acs.chemmater.7b03311 [34] YAO J S, GE J, HAN B N, et al. Ce3+-doping to modulate photoluminescence kinetics for efficient CsPbBr3 nanocrystals based light-emitting diodes[J]. Journal of the American Chemical Society, 2018, 140(10): 3626-3634. doi: 10.1021/jacs.7b11955 [35] PAN G C, BAI X, YANG D W, et al. Doping lanthanide into perovskite nanocrystals: highly improved and expanded optical properties[J]. Nano Letters, 2017, 17(12): 8005-8011. doi: 10.1021/acs.nanolett.7b04575 [36] HU Y Q, BAI F, LIU X B, et al. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells[J]. ACS Energy Letters, 2017, 2(10): 2219-2227. doi: 10.1021/acsenergylett.7b00508 [37] SAIDAMINOV M I, KIM J, JAIN A, et al. Suppression of atomic vacancies via incorporation of isovalent small ions to increase the stability of halide perovskite solar cells in ambient air[J]. Nature Energy, 2018, 3(8): 648-654. doi: 10.1038/s41560-018-0192-2 [38] LIU W Y, LIN Q L, LI H B, et al. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content[J]. Journal of the American Chemical Society, 2016, 138(45): 14954-14961. doi: 10.1021/jacs.6b08085 [39] YONG Z J, GUO SH Q, MA J P, et al. Doping-enhanced short-range order of perovskite nanocrystals for near-unity violet luminescence quantum yield[J]. Journal of the American Chemical Society, 2018, 140(31): 9942-9951. doi: 10.1021/jacs.8b04763 [40] ZHANG X T, WANG H, HU Y, et al. Strong blue emission from Sb3+-doped super small CsPbBr3 nanocrystals[J]. The Journal of Physical Chemistry Letters, 2019, 10(8): 1750-1756. doi: 10.1021/acs.jpclett.9b00790 [41] MONDAL N, DE A, SAMANTA A. Achieving near-unity photoluminescence efficiency for blue-violet-emitting perovskite nanocrystals[J]. ACS Energy Letters, 2019, 4(1): 32-39. doi: 10.1021/acsenergylett.8b01909 [42] BEHERA R K, DAS ADHIKARI S, DUTTA S K, et al. Blue-emitting CsPbCl3 nanocrystals: impact of surface passivation for unprecedented enhancement and loss of optical emission[J]. The Journal of Physical Chemistry Letters, 2018, 9(23): 6884-6891. doi: 10.1021/acs.jpclett.8b03047 [43] KOVALENKO M V, PROTESESCU L, BODNARCHUK M I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals[J]. Science, 2017, 358(6364): 745-750. doi: 10.1126/science.aam7093 [44] AKKERMAN Q A, RAINÒ G, KOVALENKO M V, et al. Genesis, challenges and opportunities for colloidal lead halide perovskite nanocrystals[J]. Nature Materials, 2018, 17(5): 394-405. doi: 10.1038/s41563-018-0018-4 [45] KIM G Y, SENOCRATE A, YANG T Y, et al. Large tunable photoeffect on ion conduction in halide perovskites and implications for photodecomposition[J]. Nature Materials, 2018, 17(5): 445-449. doi: 10.1038/s41563-018-0038-0 [46] CALADO P, TELFORD A M, BRYANT D, et al. Evidence for ion migration in hybrid perovskite solar cells with minimal hysteresis[J]. Nature Communications, 2016, 7: 13831. doi: 10.1038/ncomms13831 [47] ZHU X Y, PODZOROV V. Charge carriers in hybrid organic-inorganic lead halide perovskites might be protected as large polarons[J]. The Journal of Physical Chemistry Letters, 2015, 6(23): 4758-4761. doi: 10.1021/acs.jpclett.5b02462 [48] MIYATA K, MEGGIOLARO D, TRINH M T, et al. Large polarons in lead halide perovskites[J]. Science Advances, 2017, 3(8): 1701217. doi: 10.1126/sciadv.1701217 [49] MAHATA A, MEGGIOLARO D, DE ANGELIS F. From large to small polarons in lead, tin, and mixed lead-tin halide perovskites[J]. The Journal of Physical Chemistry Letters, 2019, 10(8): 1790-1798. doi: 10.1021/acs.jpclett.9b00422 [50] PARK M, NEUKIRCH A J, REYES-LILLO S E, et al. Excited-state vibrational dynamics toward the polaron in methylammonium lead iodide perovskite[J]. Nature Communications, 2018, 9: 2525. doi: 10.1038/s41467-018-04946-7 [51] BEAULAC R, SCHNEIDER L, ARCHER P I, et al. Light-induced spontaneous magnetization in doped colloidal quantum dots[J]. Science, 2009, 325(5943): 973-976. doi: 10.1126/science.1174419 [52] HOFFMAN D M, MEYER B K, EKIMOV A I, et al. Giant internal magnetic fields in Mn doped nanocrystal quantum dots[J]. Solid State Communications, 2000, 114(10): 547-550. doi: 10.1016/S0038-1098(00)00089-2 [53] 符靓, 施树云, 陈晓青. 电感耦合等离子体-质谱法测定高纯四甲基氢氧化铵中超痕量金属元素[J]. 分析化学,2018,46(1):107-112.FU L, SHI SH Y, CHEN X Q. Ultra-trace metal elements analysis of high purity tetramethylammonium hydroxide using inductively coupled plasma tandem mass spectrometry[J]. Chinese Journal of Analytical Chemistry, 2018, 46(1): 107-112. (in Chinese) [54] 陈文, 樊小伟, 郭才女, 等. 电感耦合等离子体串联质谱法测定高纯稀土中铁的含量[J]. 分析化学,2019,47(3):403-409.CHEN W, FAN X W, GUO C N, et al. Determination of iron content in high purity rare earth by inductively coupled plasma-tandem mass spectrometry[J]. Chinese Journal of Analytical Chemistry, 2019, 47(3): 403-409. (in Chinese) [55] WANG Y Y, WU M W. Control of spin coherence in semiconductor double quantum dots[J]. Physical Review B, 2008, 77(12): 125323. doi: 10.1103/PhysRevB.77.125323 [56] HUANG G G, WANG CH L, XU SH H, et al. Postsynthetic doping of MnCl2 molecules into preformed CsPbBr3 perovskite nanocrystals via a halide exchange-driven cation exchange[J]. Advanced Materials, 2017, 29(29): 1700095. doi: 10.1002/adma.201700095 [57] XU K Y, LIN CH CH, XIE X B, et al. Efficient and stable luminescence from Mn2+ in core and core-isocrystalline shell CsPbCl3 perovskite nanocrystals[J]. Chemistry of Materials, 2017, 29(10): 4265-4272. doi: 10.1021/acs.chemmater.7b00345 [58] LIU M, ZHONG G H, YIN Y M, et al. Aluminum-doped cesium lead bromide perovskite nanocrystals with stable blue photoluminescence used for display backlight[J]. Advanced Science, 2017, 4(11): 1700335. doi: 10.1002/advs.201700335 [59] ZHOU D L, LIU D L, PAN G CH, et al. Cerium and ytterbium codoped halide perovskite quantum dots: a novel and efficient downconverter for improving the performance of silicon solar cells[J]. Advanced Materials, 2017, 29(42): 1704149. doi: 10.1002/adma.201704149 [60] 尹子辰, 王彦玲, 张传保. 羟丙基胍胶在高岭土上的吸附性质研究[J]. 分析化学,2019,47(1):93-98.YIN Z CH, WANG Y L, ZHANG CH B. Study of adsorption behavior of hydroxypropyl guar gum on kaolin[J]. Chinese Journal of Analytical Chemistry, 2019, 47(1): 93-98. (in Chinese) [61] ISHIKAWA R, MISHRA R, LUPINI A R, et al. Direct observation of dopant atom diffusion in a bulk semiconductor crystal enhanced by a large size mismatch[J]. Physical Review Letters, 2014, 113(15): 155501. doi: 10.1103/PhysRevLett.113.155501 [62] BRENNAN M C, DRAGUTA S, KAMAT P V, et al. Light-induced anion phase segregation in mixed halide perovskites[J]. ACS Energy Letters, 2018, 3(1): 204-213. doi: 10.1021/acsenergylett.7b01151 [63] LI W, ROTHMANN M U, LIU A, et al. Phase segregation enhanced ion movement in efficient inorganic CsPbIBr2 solar cells[J]. Advanced Energy Materials, 2017, 7(20): 1700946. doi: 10.1002/aenm.201700946 [64] MIZUSAKI J, ARAI K, FUEKI K. Ionic conduction of the perovskite-type halides[J]. Solid State Ionics, 1983, 11(3): 203-211. doi: 10.1016/0167-2738(83)90025-5 [65] O’REGAN B C, BARNES P R F, LI X E, et al. Optoelectronic studies of methylammonium lead iodide perovskite solar cells with mesoporous TiO2: separation of electronic and chemical charge storage, understanding two recombination lifetimes, and the evolution of band offsets during J-V hysteresis[J]. Journal of the American Chemical Society, 2015, 137(15): 5087-5099. doi: 10.1021/jacs.5b00761 -




 下载:
下载: