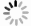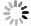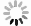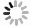Study on the binding mechanism of cefoxitin sodium to lysozyme by synchronous fluorescence spectroscopy
-
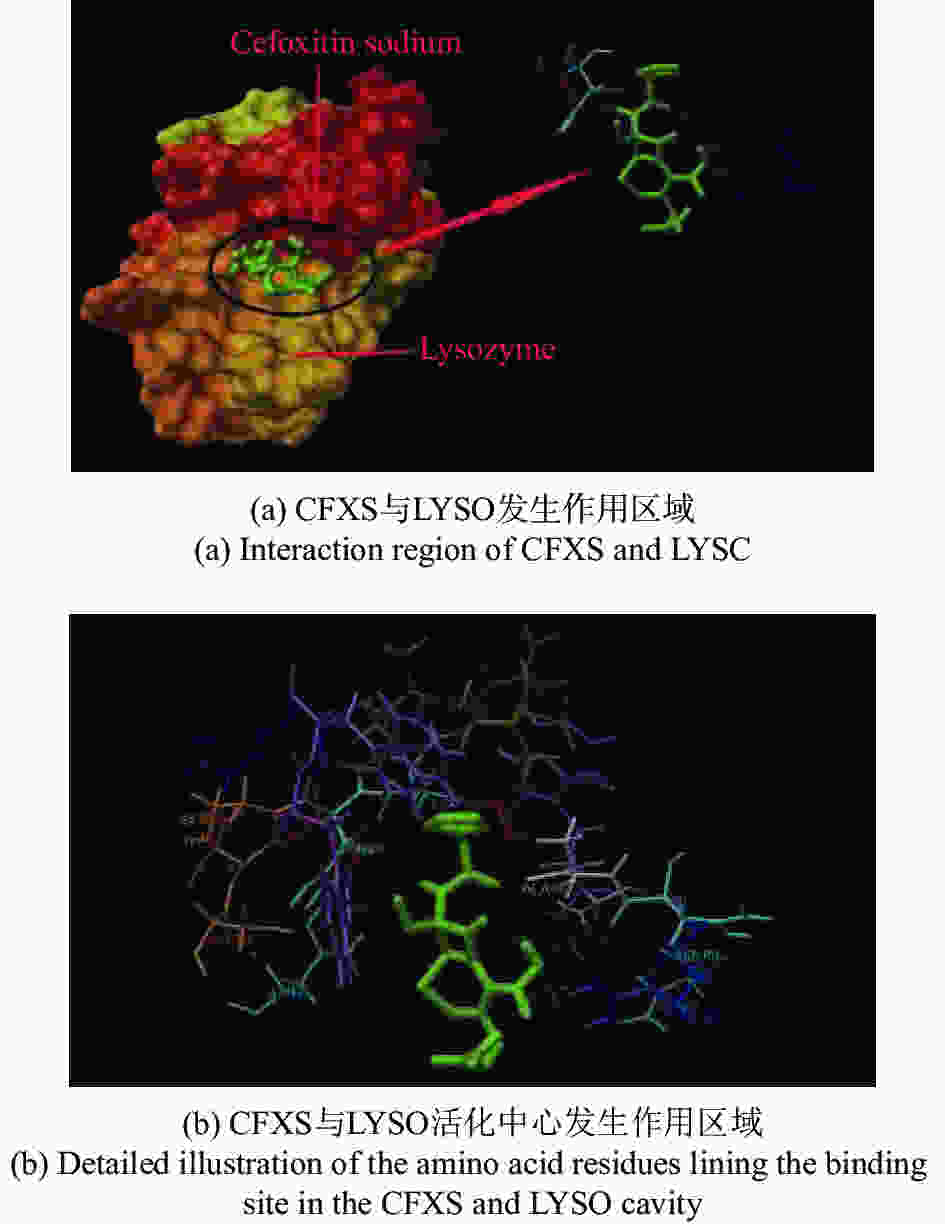
摘要: 在模拟生理学条件下(pH=7.40),采用同步荧光法研究了头孢西丁钠(CFXS)和溶菌酶(LYSO)中的荧光基团酪氨酸(Tyr)残基、色氨酸(Trp)残基之间的相互作用。结果表明:CFXS以静态猝灭的方式猝灭LYSO中的Tyr、Trp残基的荧光,结合位点数n ≈1。310 K时,Tyr与Trp残基反应的荧光猝灭比率分数NSFQR(Trp)(60.25%)>NSFQR(Tyr)(39.75%),结合位置更靠近Trp残基。Hill系数nH约为1,表明CFXS与LYSO中Tyr与Trp残基的结合不会影响后继配体与蛋白质的结合。CFXS与LYSO中Tyr残基的药物结合率W(Q)为0.19%~0.13%,Trp残基的药物结合率W(Q)为0.23%~0.14%,游离的药物含量几乎不变,这表明CFXS与LYSO中Tyr与Trp残基的结合基本不影响药物的疗效。Tyr残基的蛋白结合率W(B)为52.69%~54.67%,Trp残基的蛋白结合率W(B)为67.67%~69.39%,因此,蛋白中游离的氨基酸残基数目会明显降低。CFXS-LYSO结合体系的主要作用力类型是疏水作用,分子对接结果表明CFXS与LYSO之间还存在氢键作用,且两者的最佳结合位置在LYSO的活性中心附近,两者的结合改变了活性中心处氨基酸残基的微环境。Abstract: Under simulated physiological conditions (pH=7.40), the interaction between tyrosine (Tyr) residue and tryptophan (Trp) residue in lysozyme (LYSO) and cefoxitin sodium (CFXS) was studied using synchronous fluorescence spectroscopy. The results showed that CFXS quenched the fluorescence of Tyr and Trp residue in LYSO by static quenching, and that the number of binding sites n was nearly 1. At 310 K, the fluorescence quenching ratio of CFXS with Trp residue NSFQR(Trp)(60.25%) was higher than that of NSFQR(Tyr)(39.75%), indicating that the binding position was closer to the Trp residue. The Hill coefficient nH was about 1, indicating that the binding of CFXS to the Tyr and Trp residues in LYSO did not affect the binding of subsequent ligands to proteins. The drug binding rate of CFXS to Tyr residue in LYSO was 0.19% to 0.13%, and the drug′s binding rate to Trp residue was 0.23% to 0.14%, respectively. The content of the free drug was almost unchanged. The results showed that the combination of Tyr and Trp residue in LYSO and CFXS did not affect the efficacy of the drug. The protein binding rate of Tyr residue was 52.69% to 54.67%, and the protein binding rate of Trp residue was 67.67% to 69.39%, implying the amount of free amino acid residue in the protein decreased significantly. The main force of the CFXS-LYSO binding system was a hydrophobic interaction. The results of molecular docking showed that there was still a hydrogen bond between the CFXS and LYSO, and the best binding position was near to the active center of the LYSO. The combination of the two substances changed the microenvironment for the amino acid residue at the active center.
-
Key words:
- synchronous fluorescence /
- cefoxitin sodium /
- lysozyme /
- fluorescent group /
- molecular docking
-
表 1 CFXS-LYSO体系的同步荧光猝灭反应参数
Table 1. Reactive parameters of synchronous fluorescence quenching for CFXS-LYSO system
Δλ/nm T/K Ksv/L·mol−1·s−1 kq/L·mol−1 r1 Ka/L·mol−1 n r2 Δλ=60 298 2.18×104 2.18×1012 0.990 5 1.73×104 1.18 0.992 2 310 1.61×104 1.61×1012 0.992 4 1.58×104 1.03 0.991 3 318 1.26×104 1.26×1012 0.994 1 1.39×104 0.96 0.993 5 Δλ=15 298 1.33×104 1.33×1012 0.993 8 0.96×104 1.07 0.995 7 310 1.17×104 1.17×1012 0.991 4 0.84×104 1.17 0.992 2 318 0.83×104 0.83×1012 0.995 2 0.68×104 1.12 0.993 4 r1为方程I0 /I~[L]的线性相关系数;r2为方程lg[(I0-I)/I]~lg{[L]-n[Bt](I0-I)/I0}的线性相关系数;[Bt]=5.0×10−7 mol/L。 表 2 不同温度下CFXS-LYSO体系的热力学参数
Table 2. Thermodynamic parameters of CFXS-LYSO system at different temperatures
System T/K Ka/L·mol−1 ΔH/kJ·mol−1 ΔS/J·mol−1 K−1 ΔG/kJ·mol−1 Δλ=15 nm 298 0.96×104 −13.15 32.10 −22.71 310 0.84×104 32.70 −23.28 318 0.68×104 32.01 −23.33 Δλ=60 nm 298 1.73×104 −11.68 41.93 −24.17 310 1.58×104 42.70 −24.91 318 1.39×104 42.58 −25.22 表 3 不同温度下CFXS和LYSO中Tyr、Trp残基的Hill系数
Table 3. Hill coefficients of Tyr and Trp residues in CFXS and LYSO at different temperatures
T/K Δλ=60 nm Δλ=15 nm nH r3 nH r3 298 0.94 0.993 7 0.99 0.997 1 310 1.15 0.994 8 1.07 0.993 3 318 0.92 0.996 7 0.94 0.995 2 nH为体系的Hill系数;r3为方程lg[Y/(1-Y)]~lg[L]的线性相关系数。 表 4 CFXS-LYSO体系的对接能量(单位:kJ/mol)
Table 4. Docking energy of CFXS-LYSO system (unit: kJ/mol)
Protein PDB ID ΔG0 ΔE1 ΔE2 ΔE3 2LYZ −24.81 −39.78 −36.44 −3.34 -
[1] 张勇, 刘劲风, 易润豪, 等. 一种黄酮类荧光探针的合成及用于肼的检测[J]. 分析化学,2018,46(4):511-516.ZHANG Y, LIU J F, YI R H, et al. Synthesis and application of a flavone-based fluorescent probe for detection of hydrazine[J]. Chinese Journal of Analytical Chemistry, 2018, 46(4): 511-516. (in Chinese) [2] 曹津津, 李启瑞, 李文红, 等. 木兰花碱的荧光性质及其在中药分析中的应用研究[J]. 分析化学,2019,47(6):950-956.CAO J J, LI Q R, LI W H, et al. Fluorescence properties of magnoflorine and its application in analysis of traditional Chinese medicine[J]. Chinese Journal of Analytical Chemistry, 2019, 47(6): 950-956. (in Chinese) [3] 曹团武, 周坤, 黄文兵, 等. 光谱法研究哈巴俄苷与人血清白蛋白的结合反应[J]. 分析化学,2017,45(5):700-706. doi: 10.11895/j.issn.0253-3820.160873CAO T W, ZHOU K, HUANG W B, et al. Spectroscopic study of interaction of harpagoside and human serum albumin[J]. Chinese Journal of Analytical Chemistry, 2017, 45(5): 700-706. (in Chinese) doi: 10.11895/j.issn.0253-3820.160873 [4] 王雪荣, 朱晓静, 刘永明. 同步荧光法研究胺菊酯与牛血清白蛋白的相互作用[J]. 化学研究与应用,2010,22(6):763-766. doi: 10.3969/j.issn.1004-1656.2010.06.021WANG X R, ZHU X J, LIU Y M. Study of the interaction between tetramethrin and bovine serum album by synchronous fluorescence spectroscopy[J]. Chemical Research and Application, 2010, 22(6): 763-766. (in Chinese) doi: 10.3969/j.issn.1004-1656.2010.06.021 [5] 张源, 林哲绚, 韩溟. 同步荧光光谱法研究肿瘤芳香族氨基酸残基含量的变化[J]. 光谱学与光谱分析,2017,37(9):2822-2825.ZHANG Y, LIN ZH X, HAN M. Study on the change of the content of aromatic amino acid residues in the process of malignant transformation by synchronous fluorescence spectrometry[J]. Spectroscopy and Spectral Analysis, 2017, 37(9): 2822-2825. (in Chinese) [6] LI G X, LIU B SH, ZHANG Q J, et al. Investigation on the effect of fluorescence quenching of bovine serum albumin by cefoxitin sodium using fluorescence spectroscopy and synchronous fluorescence spectroscopy[J]. Luminescence, 2016, 31(5): 1054-1062. doi: 10.1002/bio.3071 [7] 王哲, 薛敏, 孟子晖, 等. 水凝胶纳米颗粒对溶菌酶的亲和研究[J]. 分析化学,2018,46(3):317-323. doi: 10.11895/j.issn.0253-3820.171368WANG ZH, XUE M, MENG Z H, et al. Affinity of hydrogel nanoparticles to lysozyme[J]. Chinese Journal of Analytical Chemistry, 2018, 46(3): 317-323. (in Chinese) doi: 10.11895/j.issn.0253-3820.171368 [8] 唐君, 付强, 崔勐, 等. 黄酮与溶菌酶相互作用的强度衰减-基质辅助金宝搏188软件怎么用 解吸离子化-质谱研究[J]. 分析化学,2016,44(7):1071-1076. doi: 10.11895/j.issn.0253-3820.160078TANG J, FU Q, CUI M, et al. Interaction studies of lyszoyme and flavonoids using intensity fading-matrix-assisted laser desorption/ionization-mass spectrometry[J]. Chinese Journal of Analytical Chemistry, 2016, 44(7): 1071-1076. (in Chinese) doi: 10.11895/j.issn.0253-3820.160078 [9] FANG Q, GUO CH H, WANG Y R, et al. The study on interactions between levofloxacin and model proteins by using multi-spectroscopic and molecular docking methods[J]. Journal of Biomolecular Structure and Dynamics, 2018, 36(8): 2032-2044. doi: 10.1080/07391102.2017.1341335 [10] FANG Q, WANG Y R, GUO CH H, et al. Interaction between fleroxacin and lysozyme by using multi-spectral techniques and molecular docking[J]. Spectroscopy and Spectral Analysis, 2018, 38(2): 654-659. [11] SOURAV D, GHOSH P, KOLEY S, et al. Binding of naringin and naringenin with hen egg white lysozyme: a spectroscopic investigation and molecular docking study[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2018, 192: 211-221. doi: 10.1016/j.saa.2017.11.015 [12] 陈晨, 张洪峰, 王乐, 等. 荧光光谱法研究奥沙利铂与溶菌酶的相互作用[J]. 药品评价,2015,12(4):30-33. doi: 10.3969/j.issn.1672-2809.2015.04.006CHEN CH, ZHANG H F, WANG L, et al. Studies on the interaction between oxaliplatin and lysozyme by fluorescence spectrometry[J]. Drug Evaluation, 2015, 12(4): 30-33. (in Chinese) doi: 10.3969/j.issn.1672-2809.2015.04.006 [13] GÖKOĞLU E, YILMAZ E. Fluorescence interaction and determination of sulfathiazole with trypsin[J]. Journal of Fluorescence, 2014, 24(5): 1439-1445. doi: 10.1007/s10895-014-1427-7 [14] WANG Q, ZHANG SH R, JI X H. Investigation of interaction of antibacterial drug sulfamethoxazole with human serum albumin by molecular modeling and multi-spectroscopic method[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2014, 124(8): 84-90. doi: 10.1016/j.saa.2013.12.100 [15] RAZA M, JIANG Y, WEI Y, et al. Insights from spectroscopic and in-silico techniques for the exploitation of biomolecular interactions between human serum albumin and paromomycin[J]. Colloids and Surfaces B:Biointerfaces, 2017, 157: 242-253. doi: 10.1016/j.colsurfb.2017.05.076 [16] 申炳俊, 金丽虹, 刘昱鑫, 等. 荧光光谱结合表面增强拉曼光谱法研究紫檀芪与人血清白蛋白相互作用[J]. 分析化学,2017,45(11):1613-1620. doi: 10.11895/j.issn.0253-3820.170341SHEN B J, JIN L H, LIU Y X, et al. Study of intermolecular interactions between pterostilbene and human serum albumin by fluorescence spectrometry-surface enhanced raman spectroscopy[J]. Chinese Journal of Analytical Chemistry, 2017, 45(11): 1613-1620. (in Chinese) doi: 10.11895/j.issn.0253-3820.170341 [17] 许凤杰, 彪林海, 祖元刚, 等. 丹皮酚与钙粘素相互作用的分析研究[J]. 分析化学,2017,45(7):1025-1030.XU F J, BIAO L H, ZU Y G, et al. Study on interactions between paeonol and cadherin[J]. Chinese Journal of Analytical Chemistry, 2017, 45(7): 1025-1030. (in Chinese) [18] MAKARSKA-BIALOKOZ M. Interactions of hemin with bovine serum albumin and human hemoglobin: a fluorescence quenching study[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2018, 193: 23-32. doi: 10.1016/j.saa.2017.11.063 [19] 张彦青, 刘保生. 头孢哌酮钠与牛转铁蛋白之间相互作用的光谱法与同步荧光光谱法比较研究[J]. 分析试验室,2018,37(5):503-508.ZHANG Y Q, LIU B SH. The comparison between commercial spectral method and synchronous fluorescence spectrometry on interaction of cefoperazone sodium with bovine transferrin[J]. Chinese Journal of Analysis Laboratory, 2018, 37(5): 503-508. (in Chinese) [20] NAIK K M, NANDIBEWOOR S T. Spectroscopic studies on the interaction between chalcone and bovine serum albumin[J]. Journal of Luminescence, 2013, 143: 484-491. doi: 10.1016/j.jlumin.2013.05.013 [21] SALAM A M, ROKONUJJAMAN M, RAHMAN A, et al. Study of in vitro interaction of sildenafil citrate with bovine serum albumin by fluorescence spectroscopy[J]. Pharmacology &Pharmacy, 2015, 6(2): 94-101. [22] ROSS P D, SUBRAMANIAN S. Thermodynamics of protein association reactions: forces contributing to stability[J]. Biochemistry, 1981, 20(11): 3096-3102. doi: 10.1021/bi00514a017 [23] JAHANBAN-ESFAHLAN A, PANAHI-AZAR V, SAJEDI S. Spectroscopic and molecular docking studies on the interaction between N-acetyl cysteine and bovine serum albumin[J]. Biopolymers, 2015, 103(11): 638-645. doi: 10.1002/bip.22697 [24] ZHOU H F, BI SH Y, WANG Y, et al. Characterization of the binding of paylean and DNA by fluorescence, UV spectroscopy and molecular docking techniques[J]. Luminescence, 2016, 31(4): 1013-1019. doi: 10.1002/bio.3066 [25] MA L H, LIU B SH, WANG CH D, et al. The interaction mechanism of nifedipine and pepsin[J]. Monatshefte für Chemie-Chemical Monthly, 2018, 149(11): 2123-2130. doi: 10.1007/s00706-018-2269-9 [26] REN D R, YU ZH T, ZOU Y M, et al. Enhancing the sensitivity of aptameric detection of lysozyme with a “feed-forward” network of DNA-related reaction cycles[J]. Chemistry-A European Journal, 2012, 18(44): 14201-14209. doi: 10.1002/chem.201102742 [27] 赵海雁, 高川, 郑曙光. 头孢西丁药用研究[J]. 中外健康文摘,2012,9(8):85-86. doi: 10.3969/j.issn.1672-5085.2012.08.059ZHAO H Y, GAO CH, ZHENG SH G. Cefoxitin medicinal research[J]. World Health Digest Medical Periodieal, 2012, 9(8): 85-86. (in Chinese) doi: 10.3969/j.issn.1672-5085.2012.08.059 [28] 史巧巧, 席俊, 陆启玉. 食品中苯并芘的研究进展[J]. 食品工业科技,2014,35(5):379-381, 386.SHI Q Q, XI J, LU Q Y. Research progress in benzopyrene in foods[J]. Science and Technology of Food Industry, 2014, 35(5): 379-381, 386. (in Chinese) [29] BOJKO B, SUŁKOWSKA A, MACIAŻEK-JURCZYK M, et al. The influence of dietary habits and pathological conditions on the binding of theophylline to serum albumin[J]. Journal of Pharmaceutical and Biomedical Analysis, 2010, 52(3): 384-390. doi: 10.1016/j.jpba.2009.09.004 [30] LIU B SH, WANG J, XUE CH L, et al. Spectroscopic studies on the interaction of synthetic food colorants with bovine serum albumin[J]. Zeitschrift für Physikalische Chemie, 2011, 225(4): 455-468. doi: 10.1524/zpch.2011.0070 [31] JING M Y, SONG W, LIU R T. Binding of copper to lysozyme: spectroscopic, isothermal titration calorimetry and molecular docking studies[J]. Spectrochimica Acta Part A:Molecular and Biomolecular Spectroscopy, 2016, 164: 103-109. doi: 10.1016/j.saa.2016.04.008 [32] JANA S, DALAPATI S, GHOSH S, et al. Study of microheterogeneous environment of protein human serum albumin by an extrinsic fluorescent reporter: a spectroscopic study in combination with Molecular Docking and Molecular Dynamics Simulation[J]. Journal of Photochemistry and Photobiology B:Biology, 2012, 112: 48-58. doi: 10.1016/j.jphotobiol.2012.04.007 -





 下载:
下载: