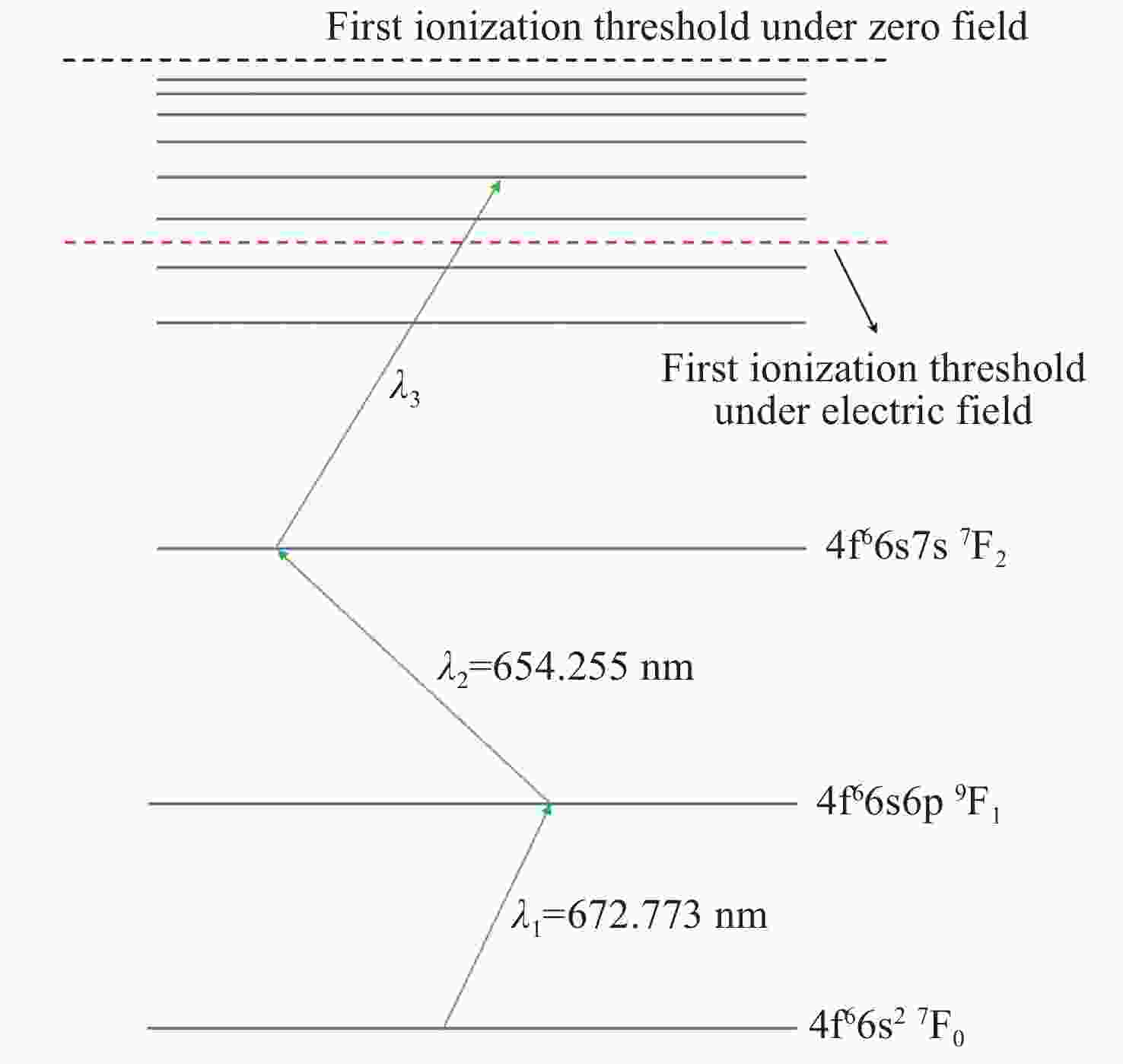
Shift of the first ionization threshold of Sm atom in electric field
doi: 10.37188/CO.2020-0071
-
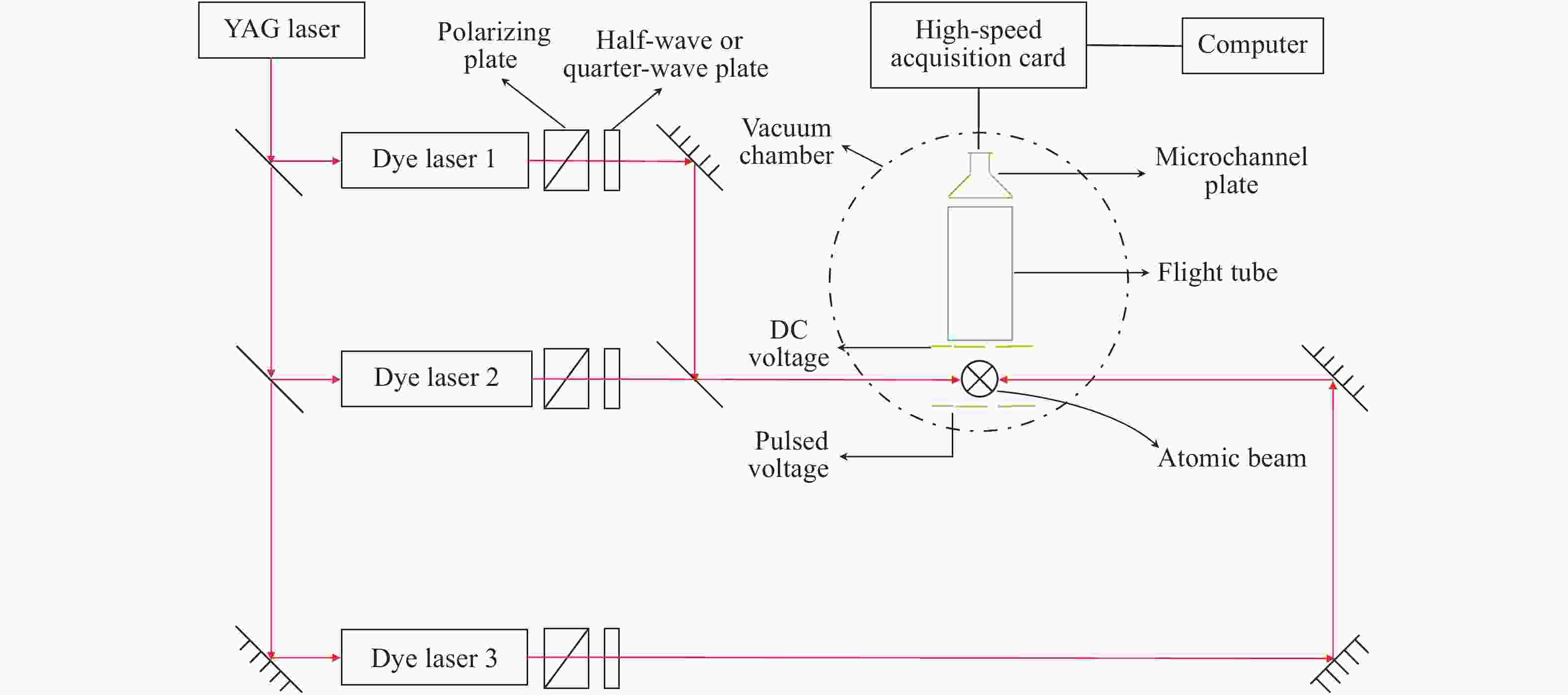
摘要: 为了获得Sm原子的第一电离阈,将Sm原子多步激发产生的光电离、自电离和场电离信号进行了区分,并研究了不同磁量子数Rydberg态Sm原子对第一电离阈的影响。首先,结合多步共振激发和偏振组合技术,将稀土Sm原子激发到第一电离阈附近具有特定磁量子数的自电离或束缚Rydberg态。接着,通过反向静电场将光电离和自电离等过程产生的离子推出作用区。然后,通过施加延时脉冲电场对束缚Rydberg态Sm原子进行探测。最后,通过改变静电场强度获得了Sm原子第一电离阈随着静电场强度的变化情况,拟合确定了零场下不同磁量子数Sm原子的第一电离阈。实验结果表明:Sm原子的第一电离阈为45519.69±0.17 cm−1;该结果与用其它方法获得的结果进行了比较。实验验证了延时场电离探测技术用于测量Sm原子第一电离阈的有效性。Abstract: In order to obtain the first ionization threshold of Sm atom, the photoionization signal, autoionization signal and field ionization signal generated by the Sm atom under multi-step excitation were distinguished, and the influence of the Rydberg state of the Sm atom with different magnetic quantum numbers on the first ionization threshold was studied. At first, by use of multi-step resonance excitation combined with polarization technology, the rare-earth Sm atoms were excited to the autoionization or bound Rydberg state with a specific magnetic quantum number near the first ionization threshold. Then the ions generated by photoionization and autoionization were pushed out of the action zone by the reverse electrostatic field, and a delayed pulsed electric field was applied to detect the Sm atoms of bound Rydberg state. Finally, the relationship between the first ionization threshold of Sm atom and the varying intensity of electrostatic field was acquired, and the first ionization threshold of the Sm atom with different magnetic quantum numbers under zero field was determined by fitting. The experimental results show that the first ionization threshold of Sm atom is 45519.69±0.17 cm−1, which has been compared with the results obtained by other methods. The effectiveness of the delayed field ionization technique in measuring the first ionization threshold of Sm atom has been verified.
-
图 6 三步金宝搏188软件怎么用 不同偏振组合下,Sm原子第一电离阈随静电场强度改变而移动的规律图。(a) π + π+ π; (b) π + π+ σ; (c) σ+ + σ+ + σ+
Figure 6. Diagram of the first ionization threshold of Sm atom shifting with the change of electrostatic field intensity under different polarization combinations of three-step lasers. (a) π + π+ π; (b) π + π+ σ; (c) σ+ + σ+ + σ+
表 1 Magnetic quantum numbers of Sm atom in 4f66snk state excited by different polarization combinations of λ1, λ2 and λ3
Table 1. Magnetic quantum numbers of Sm atom in 4f66snk state excited by different polarization combinations of λ1, λ2 and λ3
Combination
组合Polarization state
偏振状态Magnetic quantum number
磁量子数λ1 λ2 λ3 m 1 π π π 0 2 π π σ ±1 3 σ+ σ+ σ+ 3 表 2 Fitting results of the first ionization threshold of the Sm atom with different magnetic quantum numbers
Table 2. Fitting results of the first ionization threshold of the Sm atom with different magnetic quantum numbers
Combination
组合Magnetic quantum number, m
磁量子数mIonization threshold/ cm−1
电离阈/ cm−11 0 45519.67±0.21 2 ±1 45519.46±0.18 3 3 45519.95±0.11 -
[1] SATO T K, ASAI M, BORSCHEVSKY A, et al. Measurement of the first ionization potential of lawrencium, element 103[J]. Nature, 2015, 520(7546): 209-211. doi: 10.1038/nature14342 [2] CHHETRI P, ACKERMANN D, BACKE H, et al. Precision measurement of the first ionization potential of nobelium[J]. Physical Review Letters, 2018, 120(26): 263003. doi: 10.1103/PhysRevLett.120.263003 [3] RAEDER S, HEGGEN H, TEIGELHÖFER A, et al. Determination of the first ionization energy of polonium by resonance ionization spectroscopy - part I: measurement of even-parity Rydberg states at TRIUMF-ISAC[J]. Spectrochimica Acta Part B:Atomic Spectroscopy, 2019, 151: 65-71. doi: 10.1016/j.sab.2018.08.005 [4] BLOCK M. Direct mass measurements and ionization potential measurements of the actinides[J]. Radiochimica Acta, 2019, 107(9-11): 821-831. doi: 10.1515/ract-2019-3143 [5] SHEN X P, WANG W L, ZHAI L H, et al. New spectroscopic data on high-lying excited even-parity levels of atomic neodymium[J]. Spectrochimica Acta Part B:Atomic Spectroscopy, 2018, 145: 96-98. doi: 10.1016/j.sab.2018.04.012 [6] JAYASEKHARAN T, RAZVI M A N, BHALE G L. Even-parity bound and autoionizing Rydberg series of the samarium atom[J]. Journal of Physics B:Atomic,Molecular and Optical Physics, 2000, 33(16): 3123-3136. doi: 10.1088/0953-4075/33/16/314 [7] SCHMITT A, BUSHAW B A, WENDT K. Determination of the 154Sm ionization energy by high-precision laser spectroscopy[J]. Journal of Physics B:Atomic,Molecular and Optical Physics, 2004, 37(8): 1633-1644. doi: 10.1088/0953-4075/37/8/006 [8] DESCLAUX J P, FRICKE B. Relativistic prediction of the ground state of atomic Lawrencium[J]. Journal de Physique, 1980, 41(9): 943-946. doi: 10.1051/jphys:01980004109094300 [9] SHEN L, YE SH W, DAI CH J. Experiment study of ionization limit shift of europium atoms in electric fields[J]. Acta Physics Sinica, 2012, 61(6): 063301. (in Chinese) [10] WENDT K, GOTTWALD T, MATTOLAT C, et al. Ionization potentials of the lanthanides and actinides – towards atomic spectroscopy of super-heavy elements[J]. Hyperfine Interactions, 2014, 227(1): 55-67. [11] STUDER D, HEINITZ S, HEINKE R, et al. Atomic transitions and the first ionization potential of promethium determined by laser spectroscopy[J]. Physical Review A, 2019, 99(6): 062513. doi: 10.1103/PhysRevA.99.062513 [12] SHANG X, ZHOU CH X, MA L, et al. The determination of radiative lifetime for some Eu I levels by time-resolved laser-induced fluorescence spectroscopy[J]. Journal of Quantitative Spectroscopy and Radiative Transfer, 2019, 224: 103-106. doi: 10.1016/j.jqsrt.2018.11.007 [13] NIKI H, MOTOKI K, YASUI M, et al. Selectivity and efficiency of laser isotope separation processes of gadolinium[J]. Journal of Nuclear Science and Technology, 2006, 43(4): 427-431. doi: 10.1080/18811248.2006.9711117 [14] ANG'ONG'A J, GADWAY B. Polarization spectroscopy of atomic erbium in a hollow cathode lamp[J]. Journal of Physics B:Atomic,Molecular and Optical Physics, 2018, 51(4): 045003. doi: 10.1088/1361-6455/aaa1d4 [15] CHHETRI P, MOODLEY C S, RAEDER S, et al. Investigation of the first ionization potential of ytterbium in argon buffer gas[J]. Acta Physica Polonica B, 2018, 49(3): 599-603. doi: 10.5506/APhysPolB.49.599 [16] GOMONAI A I, REMETA E Y. The effect of field strength on the resonance structure of three-photon ionization spectra of the samarium atom[J]. Optics and Spectroscopy, 2013, 114(3): 329-336. doi: 10.1134/S0030400X13030119 [17] LAWLER J E, FITTANTE A J, DEN HARTOG E A. Atomic transition probabilities of neutral samarium[J]. Journal of Physics B:Atomic,Molecular and Optical Physics, 2013, 46(21): 215004. doi: 10.1088/0953-4075/46/21/215004 [18] SEEMA A U, MANDAL P K, SAHOO A C, et al.. Radiative lifetimes of even-parity high-lying levels of Sm I by delayed photoionization measurements[J]. Journal of Quantitative Spectroscopy and Radiative Transfer, 2018, 216: 1-5. [19] SAHOO A C, MANDAL P K, SHAH M L, et al. Enhancement of photoionization by applying polarization-based common level excitation scheme for the selective photoionization of odd isotopes of samarium[J]. Journal of Quantitative Spectroscopy and Radiative Transfer, 2019, 235: 7-14. doi: 10.1016/j.jqsrt.2019.06.014 [20] WU B R, XU Y F, ZHENG Y F, et al. An experimental investigation of the autoionizing levels of neutral ytterbium[J]. Journal of Physics B:Atomic,Molecular and Optical Physics, 1992, 25(2): 355-361. doi: 10.1088/0953-4075/25/2/005 [21] WORDEN E F, SOLARZ R W, PAISNER J A et al. First ionization potentials of lanthanides by laser spectroscopy[J]. Journal of the Optical Society of America, 1978, 68(1): 52-61. doi: 10.1364/JOSA.68.000052 [22] LÜ J, DAI CH J, XU Y F, et al. Perturbed 6snd 1, 3D2 rydberg series of neutral barium[J]. Chinese Physics Letters, 2001, 18(9): 1192-1195. doi: 10.1088/0256-307X/18/9/312 [23] LI Q Y, HUA L, HE M Q, et al. High-pressure photoionization/chemical ionization-time-of-flight mass spectrometry for classification and identification of green tea aromas[J]. Chinese Journal of Analytical Chemistry, 2019, 47(4): 541-549. (in Chinese) [24] HE M Q, HUA L, LI Q Y, et al. Toluene enhanced-high pressure photoionization-time-of-flight mass spectrometry for highly sensitive and rapid detection of phenolic compounds[J]. Chinese Journal of Analytical Chemistry, 2019, 47(3): 447-454. (in Chinese) [25] MARTIN W C, ZALUBAS R, HAGAN L. Atomic Energy Levels, The Rare-Earth Elements[M]. Washington: National Bureau of Standards, 1978. [26] ZHU H Q, ZHOU T, LI Y G, et al. An ultraviolet-visible absorption spectrometric method for detection of zinc (Ⅱ) and cobalt (Ⅱ) ions concentration based on boosting modeling[J]. Chinese Journal of Analytical Chemistry, 2019, 47(4): 576-582. (in Chinese) [27] ZENG K, MA Y Y, GUO Q ZH, et al. Determination of thionyl chloride residue in terephthaloyl chloride by inductively coupled plasma-optical emission spectrometry[J]. Chinese Journal of Analytical Chemistry, 2019, 47(3): 410-414. (in Chinese) [28] DAI CH J, ZHANG S, SHU X W, et al. Pulsed electric-field ionization of Stark states of neutral ytterbium[J]. Journal of Quantitative Spectroscopy and Radiative Transfer, 1995, 53(2): 179-188. doi: 10.1016/0022-4073(95)90005-5 [29] ELIZAROV A Y, CHEREPKOV N A. Two-photon polarization spectroscopy of autoionizing states[J]. Soviet Physics - JETP, 1989, 69(4): 695-699. -




 下载:
下载: