-
摘要:
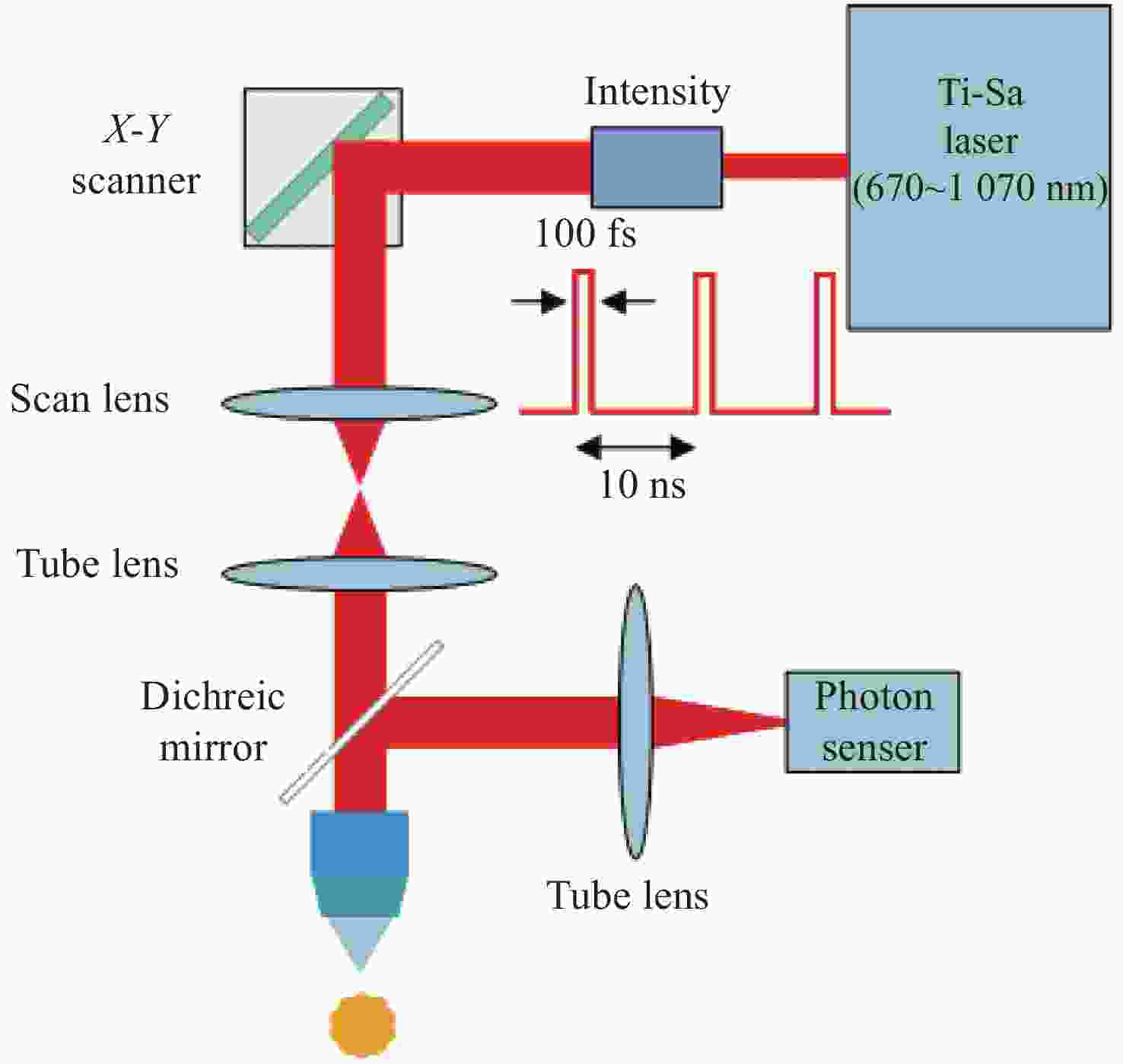
双光子显微镜在厚生物组织中依然可以保持良好的空间分辨率,这一优点使得其诞生不久就被应用于在体脑成像研究。而神经网络在时空多个维度均具有跨尺度的特点,为满足脑科学研究中在体跨尺度脑成像的需求,双光子显微镜近年来有了快速且显著的发展。本文首先介绍了双光子显微镜的工作原理,然后在成像视野、成像通量、成像深度、分辨率、微型化5个方面详细综述了双光子显微镜研究的新进展,并深入分析了跨尺度双光子在体显微成像技术的难点及未来挑战。
Abstract:Two-photon microscopy’s ability to maintain good spatial resolution in thick biological tissues has led to its application in in-vivo brain imaging studies soon after its conception. As neural networks have cross-scale multidimensional spatio-temporal properties, two-photon microscopy has developed rapidly and significantly in recent years to meet the demand for in-vivo cross-scale imaging of the brain. This paper firstly introduces the working principle of two-photon microscopy, then reviews the progress of two-photon microscopy from five perspectives: imaging field of view, imaging flux, imaging depth, resolution, miniaturization, and analyzes the difficulties and future challenges of cross-scale two-photon in-vivo microscopic imaging technology.
-
Key words:
- in-vivo brain imaging /
- cross-scale /
- two-photon microscopy
-
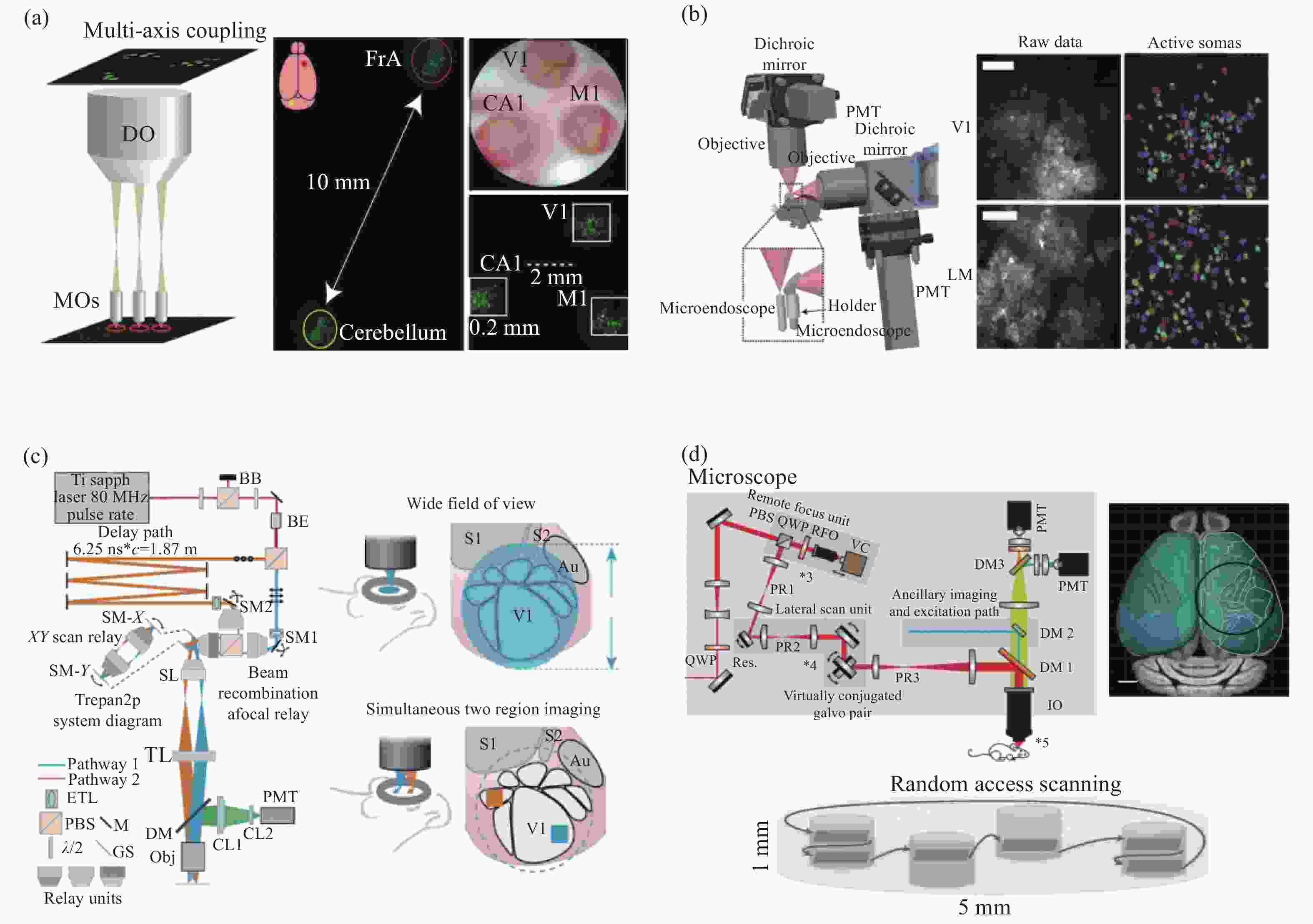
图 3 大尺度成像视野双光子显微镜。(a)多区域实时双光子成像技术[25];(b)双扫描系统双光子显微镜[26];(c)双扫描区域同步成像双光子显微镜[28];(d)多区域随机扫描双光子显微镜[31]。
Figure 3. Two-photon microscope with large-scale imaging field of view. (a) Multi-area real-time two-photon imaging technology[25]; (b) dual scanning system two-photon microscope[26]; (c) two-photon microscope with dual scanning area simultaneous imaging[28]; (d) two-photon microscope with multi-region follow-on scanning[31]
图 6 超分辨率双光子显微镜。(a)STEM成像原理和实验数据[58];(b)SIM结构光生成方法和结构光与均匀光照射探测精度对比[60]
Figure 6. Super-resolution two-photon microscope. (a) STEM imaging principles and experimental data[58]; (b) SIM structured light generation method and comparison of structured light and uniform light irradiation detection accuracy[60]
表 1 双光子显微镜在多个尺度方向的性能提升进展现状
Table 1. Progress in performance improvement of two-photon microscopy in multiple scale directions
提升的
尺度方向提升方法 结果 相关文献 成像视野 1. 使用两套或多套独立扫描探测系统;
2. 自制大视野介观物镜,双焦点扫描,脉冲延时与时分复用;
3. 自制大口径介观物镜,共振镜串联大孔径振镜,随机扫描;
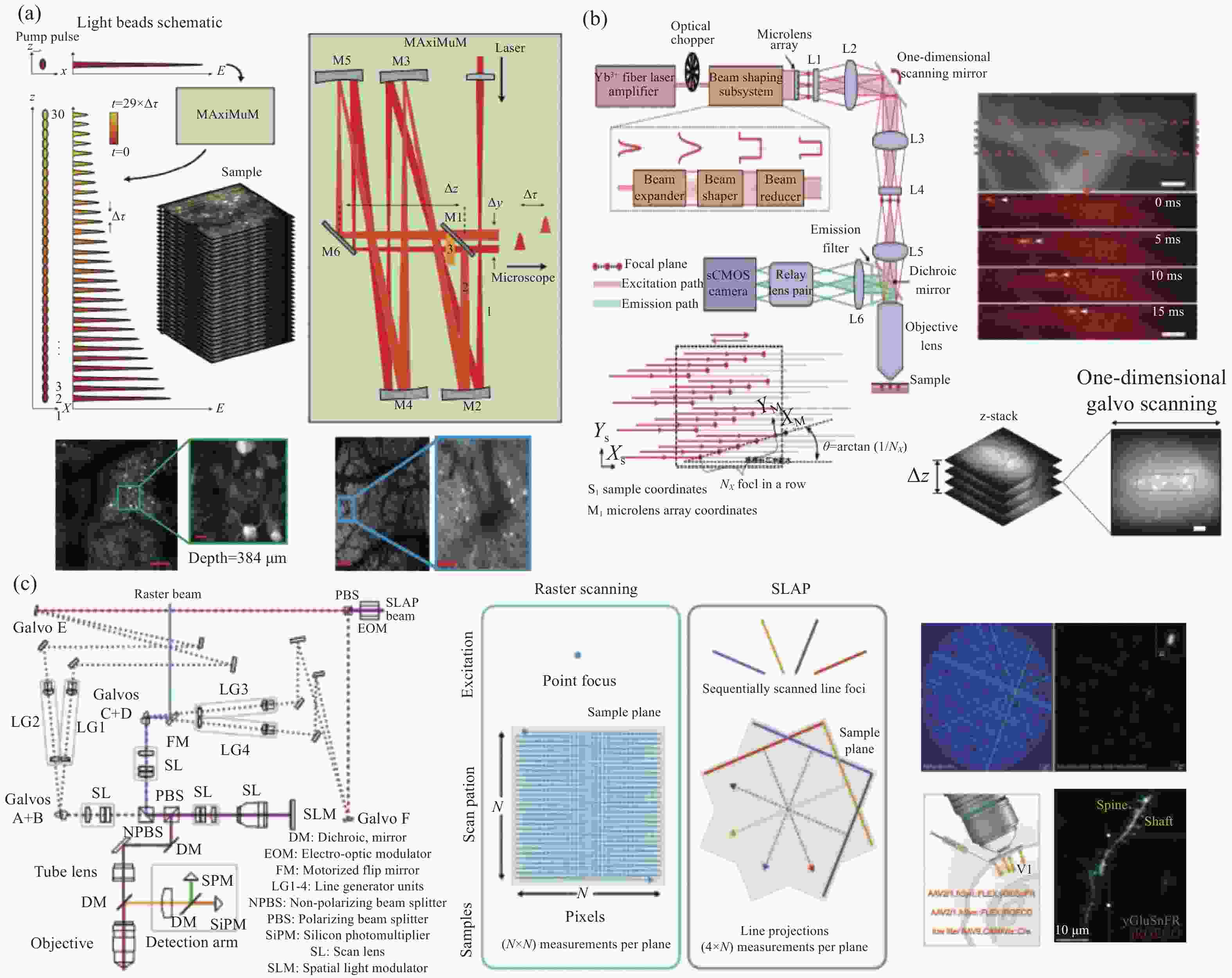
4. 使用多层阵列复合物镜结构,二次聚焦放大,多光轴耦合。将传统显微镜的成像视野直径由不到1 mm提升至12 mm。 [25−33] 成像通量 1. 光珠双光子荧光显微镜,轴向多焦点扫描;
2. 微透镜阵列将光束分束,平面多焦点扫描;
3. 线扫描,通过压缩传感算法反解出二维荧光图像。成像通量由百万量级提高至亿量级。图像帧率达到kHz量级。 [35−39] 成像深度 1. 使用更低能量(波长1300 nm)的光子,结合自适应光学,三光子激发;
2. 配合使用梯度折射率透镜,任意深度探测。成像深度由传统双光子0.7 mm提升至2.1 mm(三光子,无外源装置侵入)或任意深度(配合植入器件)。 [44−46]、[49−51] 成像分辨率 1. 受激辐射耗尽双光子荧光显微镜;
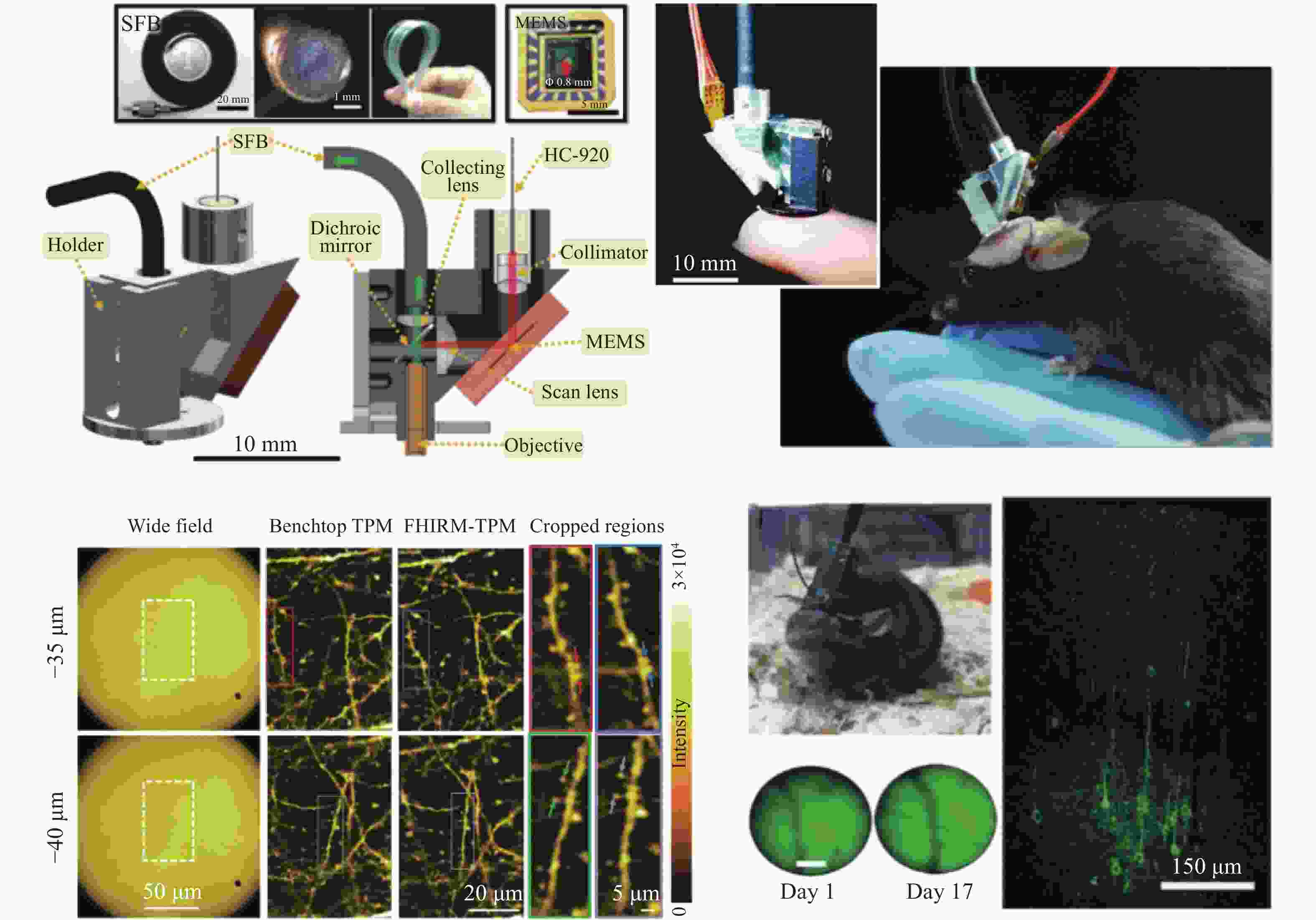
2. 结构光双光子荧光显微镜。将双光子的成像分辨率由~500 nm提升至80 nm。 [54]、[57−59]、[60−62] 微型化 1. 光纤传导激发光,MEMS、微型物镜等器件。 重量由数十 kg减少到 ~3 g;被观测动物在实验过程中可以自由移动。 [65−68] -
[1] DENK W, STRICKLER J H, WEBB W W. Two-photon laser scanning fluorescence microscopy[J]. Science, 1990, 248(4951): 73-76. doi: 10.1126/science.2321027 [2] JUVEKAR V, LEE H W, KIM H M. Two-photon fluorescent probes for detecting enzyme activities in live tissues[J]. ACS Applied Bio Materials, 2021, 4(4): 2957-2973. doi: 10.1021/acsabm.1c00063 [3] ZHANG K Y, CHEN SH, SUN H M, et al. In vivo two-photon microscopy reveals the contribution of Sox9+ cell to kidney regeneration in a mouse model with extracellular vesicle treatment[J]. Journal of Biological Chemistry, 2020, 295(34): 12203-12213. doi: 10.1074/jbc.RA120.012732 [4] ROTH R H, CUDMORE R H, TAN H L, et al. Cortical synaptic AMPA receptor plasticity during motor learning[J]. Neuron, 2020, 105(5): 895-908.E5. doi: 10.1016/j.neuron.2019.12.005 [5] YUSTE R, DENK W. Dendritic spines as basic functional units of neuronal integration[J]. Nature, 1995, 375(6533): 682-684. doi: 10.1038/375682a0 [6] DENK W, SVOBODA K. Photon upmanship: why multiphoton imaging is more than a gimmick[J]. Neuron, 1997, 18(3): 351-357. doi: 10.1016/S0896-6273(00)81237-4 [7] LICHTMAN J W, DENK W. The big and the small: challenges of imaging the brain’s circuits[J]. Science, 2011, 334(6056): 618-623. doi: 10.1126/science.1209168 [8] KANDEL E R. Principles of Neural Science[M]. New York: McGraw-Hill Medical, 2012. [9] PODOR B, HU Y L, OHKURA M, et al. Comparison of genetically encoded calcium indicators for monitoring action potentials in mammalian brain by two-photon excitation fluorescence microscopy[J]. Neurophotonics, 2015, 2(2): 021014. doi: 10.1117/1.NPh.2.2.021014 [10] VILLETTE V, CHAVARHA M, DIMOV I K, et al. Ultrafast two-photon imaging of a high-gain voltage indicator in awake behaving mice[J]. Cell, 2019, 179(7): 1590-1608.E23. doi: 10.1016/j.cell.2019.11.004 [11] DÜRST C D, OERTNER T G. Imaging Synaptic Glutamate Release with Two-Photon Microscopy in Organotypic Slice Cultures[M]//DAHLMANNS J, DAHLMANNS M. Synaptic Vesicles: Methods and Protocols. New York: Humana, 2022: 205-219. [12] KOEKKOEK L L, SLOMP M, CASTEL J, et al. Disruption of lateral hypothalamic calorie detection by a free choice high fat diet[J]. FASEB Journal, 2021, 35(9): e21804. [13] ROY R K, ALTHAMMER F, SEYMOUR A J, et al. Inverse neurovascular coupling contributes to positive feedback excitation of vasopressin neurons during a systemic homeostatic challenge[J]. Cell Reports, 2021, 37(5): 109925. doi: 10.1016/j.celrep.2021.109925 [14] LICHTMAN J W, CONCHELLO J A. Fluorescence microscopy[J]. Nature Methods, 2005, 2(12): 910-919. doi: 10.1038/nmeth817 [15] ZIPFEL W R, WILLIAMS R M, WEBB W W. Nonlinear magic: multiphoton microscopy in the biosciences[J]. Nature Biotechnology, 2003, 21(11): 1369-1377. doi: 10.1038/nbt899 [16] XU C, WEBB W W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm[J]. Journal of the Optical Society of America B, 1996, 13(3): 481-491. doi: 10.1364/JOSAB.13.000481 [17] HELMCHEN F, DENK W. Deep tissue two-photon microscopy[J]. Nature Methods, 2005, 2(12): 932-940. doi: 10.1038/nmeth818 [18] SVOBODA K, BLOCK S M. Biological applications of optical forces[J]. Annual Review of Biophysics and Biomolecular Structure, 1994, 23: 247-285. doi: 10.1146/annurev.bb.23.060194.001335 [19] SQUIRRELL J M, WOKOSIN D L, WHITE J G, et al. Long-term two-photon fluorescence imaging of mammalian embryos without compromising viability[J]. Nature Biotechnology, 1999, 17(8): 763-767. doi: 10.1038/11698 [20] SO P T, DONG C Y, MASTERS B R, et al. Two-photon excitation fluorescence microscopy[J]. Annual Review of Biomedical Engineering, 2000, 2: 399. doi: 10.1146/annurev.bioeng.2.1.399 [21] PATTERSON G H, PISTON D W. Photobleaching in two-photon excitation microscopy[J]. Biophysical Journal, 2000, 78(4): 2159-2162. doi: 10.1016/S0006-3495(00)76762-2 [22] HOPT A, NEHER E. Highly nonlinear photodamage in two-photon fluorescence microscopy[J]. Biophysical Journal, 2001, 80(4): 2029-2036. doi: 10.1016/S0006-3495(01)76173-5 [23] KUCHIBHOTLA K V, GILL J V, LINDSAY G W, et al. Parallel processing by cortical inhibition enables context-dependent behavior[J]. Nature Neuroscience, 2017, 20(1): 62-71. doi: 10.1038/nn.4436 [24] STRINGER C, PACHITARIU M, STEINMETZ N, et al. High-dimensional geometry of population responses in visual cortex[J]. Nature, 2019, 571(7765): 361-365. doi: 10.1038/s41586-019-1346-5 [25] YANG M K, ZHOU ZH Q, ZHANG J X, et al. MATRIEX imaging: multiarea two-photon real-time in vivo explorer[J]. Light:Science &Applications, 2019, 8: 109. [26] LECOQ J, SAVALL J, VUČINIĆ D, et al. Visualizing mammalian brain area interactions by dual-axis two-photon calcium imaging[J]. Nature Neuroscience, 2014, 17(12): 1825-1829. doi: 10.1038/nn.3867 [27] KIM T H, SCHNITZER M J. Fluorescence imaging of large-scale neural ensemble dynamics[J]. Cell, 2022, 185(1): 9-41. doi: 10.1016/j.cell.2021.12.007 [28] STIRMAN J N, SMITH I T, KUDENOV M W, et al. Wide field-of-view, multi-region, two-photon imaging of neuronal activity in the mammalian brain[J]. Nature Biotechnology, 2016, 34(8): 857-862. doi: 10.1038/nbt.3594 [29] CLOUGH M, CHEN I A, PARK S W, et al. Flexible simultaneous mesoscale two-photon imaging of neural activity at high speeds[J]. Nature Communications, 2021, 12(1): 6638. doi: 10.1038/s41467-021-26737-3 [30] YU CH H, STIRMAN J N, YU Y Y, et al. Diesel2p mesoscope with dual independent scan engines for flexible capture of dynamics in distributed neural circuitry[J]. Nature Communications, 2021, 12(1): 6639. doi: 10.1038/s41467-021-26736-4 [31] SOFRONIEW N J, FLICKINGER D, KING J, et al. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging[J]. eLife, 2016, 5: e14472. doi: 10.7554/eLife.14472 [32] YAO J, GAO Y F, YIN Y X, et al. Exploiting the potential of commercial objectives to extend the field of view of two-photon microscopy by adaptive optics[J]. Optics Letters, 2022, 47(4): 989-992. doi: 10.1364/OL.450973 [33] 姚靖, 吴婷, 叶世蔚, 等. 离轴抛物镜扫描中继系统提升双光子显微成像视场[J]. 金宝搏188软件怎么用 生物学报,2020,29(3):217-224. doi: 10.3969/j.issn.1007-7146.2020.03.004YAO J, WU T, YE SH W, et al. Off-axis parabolic mirror afocal scanning system extends the imaging area of two-photon microscopy[J]. Acta Laser Biology Sinica, 2020, 29(3): 217-224. (in Chinese) doi: 10.3969/j.issn.1007-7146.2020.03.004 [34] PAPAGIAKOUMOU E, RONZITTI E, EMILIANI V. Scanless two-photon excitation with temporal focusing[J]. Nature Methods, 2020, 17(6): 571-581. doi: 10.1038/s41592-020-0795-y [35] GAO Y F, XIA X Y, LIU L N, et al. Axial gradient excitation accelerates volumetric imaging of two-photon microscopy[J]. Photonics Research, 2022, 10(3): 687-696. doi: 10.1364/PRJ.441778 [36] SONG A, CHARLES A S, KOAY S A, et al. Volumetric two-photon imaging of neurons using stereoscopy (vTwINS)[J]. Nature Methods, 2017, 14(4): 420-426. doi: 10.1038/nmeth.4226 [37] DEMAS J, MANLEY J, TEJERA F, et al. High-speed, cortex-wide volumetric recording of neuroactivity at cellular resolution using light beads microscopy[J]. Nature Methods, 2021, 18(9): 1103-1111. doi: 10.1038/s41592-021-01239-8 [38] ZHANG T, HERNANDEZ O, CHRAPKIEWICZ R, et al. Kilohertz two-photon brain imaging in awake mice[J]. Nature Methods, 2019, 16(11): 1119-1122. doi: 10.1038/s41592-019-0597-2 [39] KAZEMIPOUR A, NOVAK O, FLICKINGER D, et al. Kilohertz frame-rate two-photon tomography[J]. Nature Methods, 2019, 16(8): 778-786. doi: 10.1038/s41592-019-0493-9 [40] WU J L, LIANG Y J, CHEN SH, et al. Kilohertz two-photon fluorescence microscopy imaging of neural activity in vivo[J]. Nature Methods, 2020, 17(3): 287-290. doi: 10.1038/s41592-020-0762-7 [41] WU J L, XU Y Q, XU J J, et al. Ultrafast laser-scanning time-stretch imaging at visible wavelengths[J]. Light:Science &Applications, 2017, 6(1): e16196. [42] KARPF S, RICHE C T, DI CARLO D, et al. Spectro-temporal encoded multiphoton microscopy and fluorescence lifetime imaging at kilohertz frame-rates[J]. Nature Communications, 2020, 11(1): 2062. doi: 10.1038/s41467-020-15618-w [43] JUNG J C, MEHTA A D, AKSAY E, et al. In vivo mammalian brain imaging using one- and two-photon fluorescence microendoscopy[J]. Journal of Neurophysiology, 2004, 92(5): 3121-3133. doi: 10.1152/jn.00234.2004 [44] BOCARSLY M E, JIANG W CH, WANG CH, et al. Minimally invasive microendoscopy system for in vivo functional imaging of deep nuclei in the mouse brain[J]. Biomedical Optics Express, 2015, 6(11): 4546-4556. doi: 10.1364/BOE.6.004546 [45] ANTONINI A, SATTIN A, MORONI M, et al. Extended field-of-view ultrathin microendoscopes for high-resolution two-photon imaging with minimal invasiveness[J]. eLife, 2020, 9: e58882. doi: 10.7554/eLife.58882 [46] QIN ZH Y, CHEN C P, HE S C, et al. Adaptive optics two-photon endomicroscopy enables deep-brain imaging at synaptic resolution over large volumes[J]. Science Advances, 2020, 6(40): eabc6521. doi: 10.1126/sciadv.abc6521 [47] OHEIM M, BEAUREPAIRE E, CHAIGNEAU E, et al. Two-photon microscopy in brain tissue: parameters influencing the imaging depth[J]. Journal of Neuroscience Methods, 2001, 111(1): 29-37. doi: 10.1016/S0165-0270(01)00438-1 [48] THEER P, HASAN M T, DENK W. Two-photon imaging to a depth of 1000 µm in living brains by use of a Ti∶Al2O3 regenerative amplifier[J]. Optics Letters, 2003, 28(12): 1022-1024. doi: 10.1364/OL.28.001022 [49] STREICH L, BOFFI J C, WANG L, et al. High-resolution structural and functional deep brain imaging using adaptive optics three-photon microscopy[J]. Nature Methods, 2021, 18(10): 1253-1258. doi: 10.1038/s41592-021-01257-6 [50] JI N. Adaptive optical fluorescence microscopy[J]. Nature Methods, 2017, 14(4): 374-380. doi: 10.1038/nmeth.4218 [51] LIU H J, DENG X Q, TONG SH, et al. In vivo deep-brain structural and hemodynamic multiphoton microscopy enabled by quantum dots[J]. Nano Letters, 2019, 19(8): 5260-5265. doi: 10.1021/acs.nanolett.9b01708 [52] INAVALLI V V G K, LENZ M O, BUTLER C, et al. A super-resolution platform for correlative live single-molecule imaging and STED microscopy[J]. Nature Methods, 2019, 16(12): 1263-1268. doi: 10.1038/s41592-019-0611-8 [53] FÜRSTENBERG A, HEILEMANN M. Single-molecule localization microscopy-near-molecular spatial resolution in light microscopy with photoswitchable fluorophores[J]. Physical Chemistry Chemical Physics, 2013, 15(36): 14919-14930. doi: 10.1039/c3cp52289j [54] REGO E H, SHAO L. Practical Structured Illumination Microscopy[M]//VERVEER P J. Advanced Fluorescence Microscopy: Methods and Protocols. New York: Humana Press, 2015: 175-192. [55] SCHRADER M, MEINECKE F, BAHLMANN K, et al. Monitoring the excited state of a fluorophore in a microscope by stimulated emission[J]. Bioimaging, 1995, 3(4): 147-153. doi: 10.1002/1361-6374(199512)3:4<147::AID-BIO1>3.0.CO;2-H [56] RITTWEGER E, HAN K Y, IRVINE S E, et al. STED microscopy reveals crystal colour centres with nanometric resolution[J]. Nature Photonics, 2009, 3(3): 144-147. doi: 10.1038/nphoton.2009.2 [57] BIANCHINI P, DIASPRO A. Fast scanning STED and two-photon fluorescence excitation microscopy with continuous wave beam[J]. Journal of Microscopy, 2012, 245(3): 225-228. doi: 10.1111/j.1365-2818.2011.03577.x [58] BIANCHINI P, HARKE B, GALIANI S, et al. Single-wavelength two-photon excitation–stimulated emission depletion (SW2PE-STED) superresolution imaging[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(17): 6390-6393. doi: 10.1073/pnas.1119129109 [59] LI Y, LIU SH J, SUN D Q, et al. Single-layer multitasking vortex-metalens for ultra-compact two-photon excitation STED endomicroscopy imaging[J]. Optics Express, 2021, 29(3): 3795-3807. doi: 10.1364/OE.416698 [60] LI Z W, HOU J, SUO J L, et al. Contrast and resolution enhanced optical sectioning in scattering tissue using line-scanning two-photon structured illumination microscopy[J]. Optics Express, 2017, 25(25): 32010-32020. doi: 10.1364/OE.25.032010 [61] URBAN B E, YI J, CHEN S Y, et al. Super-resolution two-photon microscopy via scanning patterned illumination[J]. Physical Review E, 2015, 91(4): 042703. [62] ZHENG W, WU Y C, WINTER P, et al. Adaptive optics improves multiphoton super-resolution imaging[J]. Nature Methods, 2017, 14(9): 869-872. doi: 10.1038/nmeth.4337 [63] SUN SH Y, HE M F, ZHANG ZH M, et al. Enhancing the axial resolution of two-photon imaging[J]. Applied Optics, 2019, 58(18): 4892-4897. doi: 10.1364/AO.58.004892 [64] YE SH W, YIN Y X, YAO J, et al. Axial resolution improvement of two-photon microscopy by multi-frame reconstruction and adaptive optics[J]. Biomedical Optics Express, 2020, 11(11): 6634-6648. doi: 10.1364/BOE.409651 [65] ZONG W J, WU R L, LI M L, et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice[J]. Nature Methods, 2017, 14(7): 713-719. doi: 10.1038/nmeth.4305 [66] OZBAY B N, FUTIA G L, MA M, et al. Three dimensional two-photon brain imaging in freely moving mice using a miniature fiber coupled microscope with active axial-scanning[J]. Scientific Reports, 2018, 8(1): 8108. doi: 10.1038/s41598-018-26326-3 [67] HENDRIKS B H W, KUIPER S, VAN AS M A J, et al. Electrowetting-based variable-focus lens for miniature systems[J]. Optical Review, 2005, 12(3): 255-259. doi: 10.1007/s10043-005-0255-z [68] ZONG W J, OBENHAUS H A, SKYTØEN E R, et al. Large-scale two-photon calcium imaging in freely moving mice[J]. Cell, 2022, 185(7): 1240-1256.E30. doi: 10.1016/j.cell.2022.02.017 [69] LOTT G E, MARCINIAK M A, BURKE J H. Three-dimensional imaging of trapped cold atoms with a light field microscope[J]. Applied Optics, 2017, 56(31): 8738-8745. doi: 10.1364/AO.56.008738 [70] STEFANOIU A, SCROFANI G, SAAVEDRA G, et al. What about computational super-resolution in fluorescence Fourier light field microscopy?[J]. Optics Express, 2020, 28(11): 16554-16568. doi: 10.1364/OE.391189 -






 下载:
下载: