-
摘要:
光学显微成像技术具有实时性、高分辨率和非侵入性等特点,其成像尺度可跨越细胞、组织乃至生命体,极大地拓展了人们对生命本质的认识边界。然而,受限于光学显微成像系统有限的空间带宽积(Space-Bandwidth Product,SBP),常规的光学显微镜难以同时兼具大视场和高分辨率,使得显微成像在大视场生物成像应用中受到较大的限制,例如,对脑神经网络以突触为单位的神经回路成像。近年来,大视场光学显微成像技术得到不断的发展,其SBP的视场相较于传统的光学显微镜有了十倍甚至百倍的提升,在保持高分辨率的基础上拓展了成像视场,从而可以满足生物医学领域重大问题的研究需求。本文介绍了近年来几种典型的大视场光学显微成像技术及其生物医学应用,并对其未来发展做了展望。
Abstract:With the characteristics of real-time, high-resolution and non-invasive, optical microscopy can scale from cells, tissues to whole living organisms, which has greatly expanded our understanding to the nature of life. However, due to the limited Space-Bandwidth Product (SBP), it is hard for a conventional optical microscope to achieve a large field of view with a high resolution. This makes it very difficult for microscopic imaging in large field of view biological imaging applications, such as imaging of neural circuits between the synapse of the brain neural networks. Recently, large field-of-view imaging technology has received increasing attention and experienced rapid development. The SBP has been improved ten times or even a hundred times as compared to a traditional optical microscope and the field-of-view has been expanded without sacrificing resolution, which, in turn, has resolved some major problems in biomedical research. This review introduces the progress, characteristics and corresponding biological applications of several typical trans-scale optical imaging techniques in recent years, and gives an outlook on their future development.
-
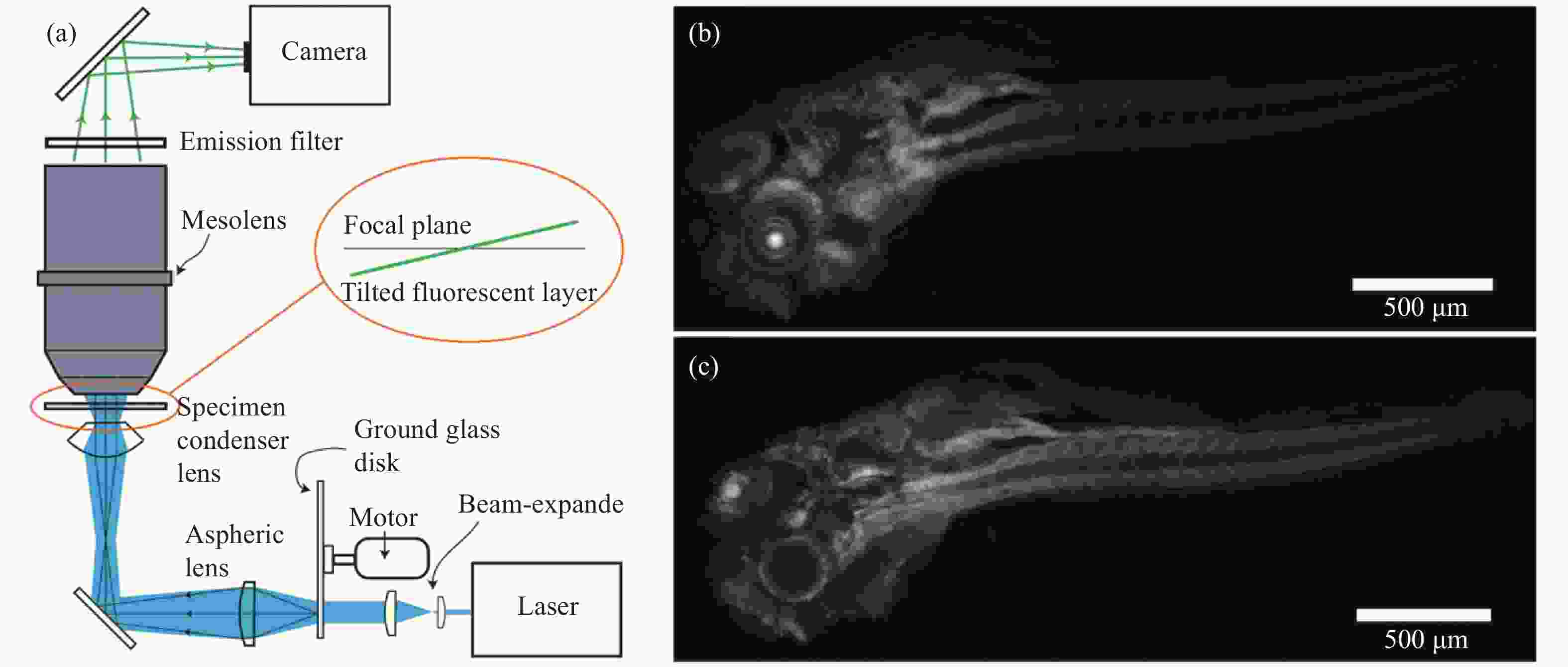
图 1 大视场物镜双光子脑成像[12]。(a)成像系统光路图和物镜实物图(左上);(b) 活体鼠脑神经细胞的双光子成像,深度为150 μm;(c)图(b)中虚线框内的细节放大图
Figure 1. Large FOV objective two-photon brain imaging[12]. (a) Optical path diagram of the imaging system and objective lens (upper left); (b) two-photon imaging of neuronal cells activity of mice brain in vivo, the depth of imaging is 150 μm; (c) magnification of some details in white box of (b)
图 2 基于散斑光片照明的大视场成像系统[25]。(a) 系统光路示意图,金宝搏188软件怎么用 经磨砂玻璃片形成的散斑图案投影在体积为4.4 mm×3 mm×3 mm的样品内,形成厚度约为3 μm的散斑光片照明;(b) 用光片照明模式实现斑马鱼全身成像;(c)用共聚焦模式实现的斑马鱼全身成像
Figure 2. Speckle light sheet illumination-based large FOV imaging system[25]. (a) System setting, the speckle pattern formed by the laser through the ground glass disk is projected into the sample with a volume of 4.4 mm×3 mm×3 mm, forming a speckle light sheet with a thickness of about 3 μm for illumination; (b) Zebrafish whole body imaging with light sheet illumination; (c) Zebrafish whole body imaging with confocal microscopy
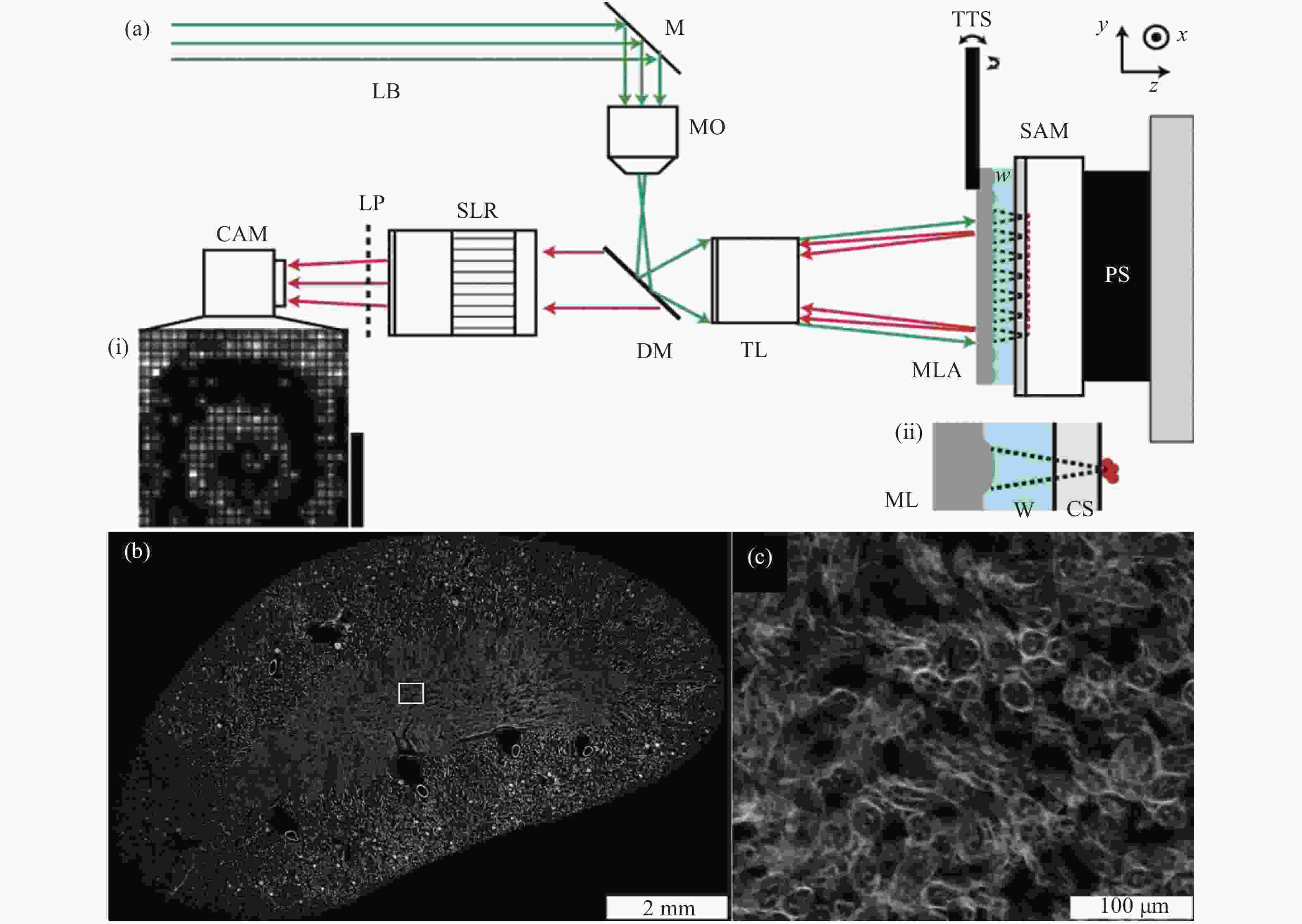
图 3 微透镜的排列与扫描方向示意图[37]
Figure 3. Schematic diagram of microlenses arrangement and scanning direction
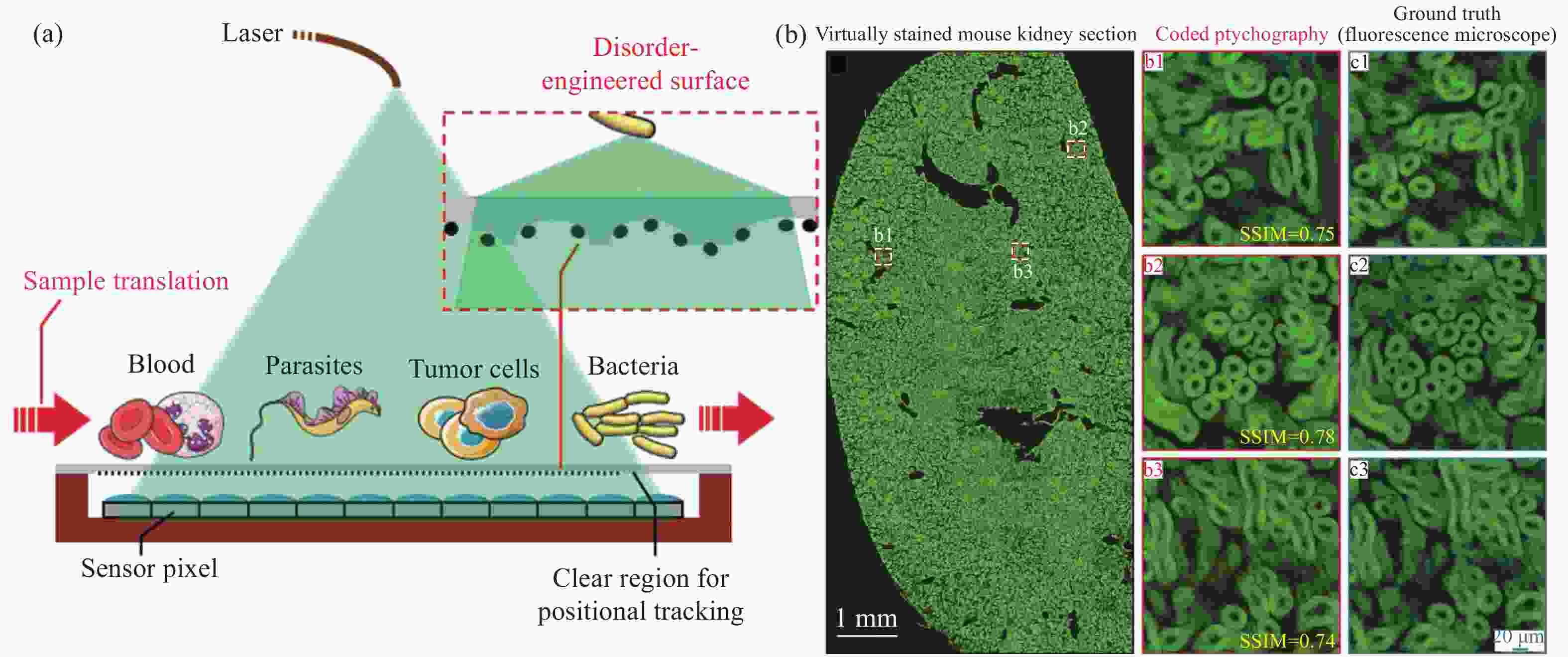
图 5 无透镜分辨率增强成像系统[21]。(a) 系统结构和其使用的无序表面示意图;(b) 系统对小鼠肾脏切片的高通量成像,并选取三个子区域b1, b2, b3(图中红色框内)的成像结果与使用20 X, 0.75 NA物镜的荧光显微镜成像结果做对比,两者的相似度约为0.75
Figure 5. Resolution enhanced lensless imaging system[21]. (a) Schematic diagram of system design and the disordered surface; (b) high-throughput imaging of mouse kidney slices. Imaging results of three sub-regions b1, b2, b3 (red boxes) are compared with that of the fluorescence microscope using an objective with 20 X and 0.75 NA. The similarity is about 0.75
表 1 4类典型的大视场光学显微成像技术参数对比
Table 1. Comparison of four representative optical microscopy imaging techniques with large FOV
技术类别 成像方式 分辨率/μm 帧率(frame·s−1) 视场 优缺点 适用场景 大视场物镜成像 宽场[27] 0.7 92 20 mm2 可兼容多种成像方式;
像质分布不均匀活体、细胞、切片观察 双光子[17,27] 0.6 5×10−3 20 mm2 光片[29] 0.7 0.15 20 mm2 曲面探测成像 串行成像[37] 1.5 0.7 1256 mm2 整体像质更好;成像方式
多局限于宽场活体、细胞、切片观察 并行成像[19] 1.2 30 113 mm2 阵列显微 宽场+扫描[41] 1.7 6 60 mm2 简单;焦深浅 切片观测 无透镜显微 多波长复用[53] 0.69 0.5 29 mm2 简单,低成本;成像保真度有限 细胞、切片观测 倾斜成像[55] 0.69 5.6×10−2 120 mm2 编码叠层成像[26] 0.3 6.7×10−2 240 mm2 -
[1] PARK J, BRADY D J, ZHENG G A, et al. Review of bio-optical imaging systems with a high space-bandwidth product[J]. Advanced Photonics, 2021, 3(4): 044001. [2] GUSTAFSSON M G L, AGARD D A, SEDAT J W. Doubling the lateral resolution of wide-field fluorescence microscopy using structured illumination[J]. Proceedings of SPIE, 2000, 3919: 141-150. doi: 10.1117/12.384189 [3] GUSTAFSSON M G L. Nonlinear structured-illumination microscopy: wide-field fluorescence imaging with theoretically unlimited resolution[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(37): 13081-13086. doi: 10.1073/pnas.0406877102 [4] ZHENG G A, SHEN CH, JIANG SH W, et al. Concept, implementations and applications of Fourier ptychography[J]. Nature Reviews Physics, 2021, 3(3): 207-223. doi: 10.1038/s42254-021-00280-y [5] BIAN Z CH, GUO CH F, JIANG SH W, et al. Autofocusing technologies for whole slide imaging and automated microscopy[J]. Journal of Biophotonics, 2020, 13(12): e202000227. [6] TSAI P S, MATEO C, FIELD J J, et al. Ultra-large field-of-view two-photon microscopy[J]. Optics Express, 2015, 23(11): 13833-13847. doi: 10.1364/OE.23.013833 [7] OLIVAS S J, ARIANPOUR A, STAMENOV I, et al. Image processing for cameras with fiber bundle image relay[J]. Applied Optics, 2015, 54(5): 1124-1137. doi: 10.1364/AO.54.001124 [8] GREENBAUM A, LUO W, SU T W, et al. Imaging without lenses: achievements and remaining challenges of wide-field on-chip microscopy[J]. Nature Methods, 2012, 9(9): 889-895. doi: 10.1038/nmeth.2114 [9] FARAHANI N, PARWANI A, PANTANOWITZ L. Whole slide imaging in pathology: advantages, limitations, and emerging perspectives[J]. Pathology and Laboratory Medicine International, 2015, 2015(7): 23-33. [10] BARISONI L, LAFATA K J, HEWITT S M, et al. Digital pathology and computational image analysis in nephropathology[J]. Nature Reviews Nephrology, 2020, 16(11): 669-685. doi: 10.1038/s41581-020-0321-6 [11] ZHENG G A, OU X Z, YANG C. 0.5 gigapixel microscopy using a flatbed scanner[J]. Biomedical Optics Express, 2014, 5(1): 1-8. doi: 10.1364/BOE.5.000001 [12] SOFRONIEW N J, FLICKINGER D, KING J, et al. A large field of view two-photon mesoscope with subcellular resolution for in vivo imaging[J]. eLife, 2016, 5: e14472. doi: 10.7554/eLife.14472 [13] PACHECO S, WANG CH L, CHAWLA M K, et al. High resolution, high speed, long working distance, large field of view confocal fluorescence microscope[J]. Scientific Reports, 2017, 7(1): 13349. doi: 10.1038/s41598-017-13778-2 [14] FAN J T, SUO J L, WU J M, et al. Video-rate imaging of biological dynamics at centimetre scale and micrometre resolution[J]. Nature Photonics, 2019, 13(11): 809-816. doi: 10.1038/s41566-019-0474-7 [15] WEINSTEIN R S, DESCOUR M R, LIANG CH, et al. An array microscope for ultrarapid virtual slide processing and telepathology. Design, fabrication, and validation study[J]. Human Pathology, 2004, 35(11): 1303-1314. doi: 10.1016/j.humpath.2004.09.002 [16] ORTH A, CROZIER K B. High throughput multichannel fluorescence microscopy with microlens arrays[J]. Optics Express, 2014, 22(15): 18101-18112. doi: 10.1364/OE.22.018101 [17] SON J, MANDRACCHIA B, JIA SH. Miniaturized modular-array fluorescence microscopy[J]. Biomedical Optics Express, 2020, 11(12): 7221-7235. doi: 10.1364/BOE.410605 [18] HARDIE R C, BARNARD K J, BOGNAR J G, et al. High-resolution image reconstruction from a sequence of rotated and translated frames and its application to an infrared imaging system[J]. Optical Engineering, 1998, 37(1): 247-260. doi: 10.1117/1.601623 [19] COSKUN A F, SENCAN I, SU T W, et al. Lensless wide-field fluorescent imaging on a chip using compressive decoding of sparse objects[J]. Optics Express, 2010, 18(10): 10510-10523. doi: 10.1364/OE.18.010510 [20] ZHANG Y B, ALEXANDER M, YANG S, et al. High-throughput screening of encapsulated islets using wide-field lens-free on-chip imaging[J]. ACS Photonics, 2018, 5(6): 2081-2086. doi: 10.1021/acsphotonics.8b00343 [21] JIANG SH W, GUO CH F, SONG P M, et al. Resolution-enhanced parallel coded ptychography for high-throughput optical imaging[J]. ACS Photonics, 2021, 8(11): 3261-3271. doi: 10.1021/acsphotonics.1c01085 [22] MCCONNELL G, TRÄGÅRDH J, AMOR R, et al. A novel optical microscope for imaging large embryos and tissue volumes with sub-cellular resolution throughout[J]. eLife, 2016, 5: e18659. doi: 10.7554/eLife.18659 [23] JONKMAN J, BROWN C M, WRIGHT G D, et al. Tutorial: guidance for quantitative confocal microscopy[J]. Nature Protocols, 2020, 15(5): 1585-1611. doi: 10.1038/s41596-020-0313-9 [24] POWER R M, HUISKEN J. A guide to light-sheet fluorescence microscopy for multiscale imaging[J]. Nature Methods, 2017, 14(4): 360-373. doi: 10.1038/nmeth.4224 [25] SCHNIETE J, FRANSSEN A, DEMPSTER J, et al. Fast optical sectioning for widefield fluorescence mesoscopy with the mesolens based on HiLo microscopy[J]. Scientific Reports, 2018, 8(1): 16259. doi: 10.1038/s41598-018-34516-2 [26] PERON S P, FREEMAN J, IYER V, et al. A cellular resolution map of barrel cortex activity during tactile behavior[J]. Neuron, 2015, 86(3): 783-799. doi: 10.1016/j.neuron.2015.03.027 [27] SOFRONIEW N J, VLASOV Y A, HIRES S A, et al. Neural coding in barrel cortex during whisker-guided locomotion[J]. eLife, 2015, 4: 12559. doi: 10.7554/eLife.12559 [28] JI N, FREEMAN J, SMITH S L. Technologies for imaging neural activity in large volumes[J]. Nature Neuroscience, 2016, 19(9): 1154-1164. doi: 10.1038/nn.4358 [29] LIN P D, JOHNSON R B. Seidel aberration coefficients: an alternative computational method[J]. Optics Express, 2019, 27(14): 19712-19725. doi: 10.1364/OE.27.019712 [30] GRAYSON T P. Curved focal plane wide-field-of-view telescope design[J]. Proceedings of SPIE, 2002, 4849: 269-275. doi: 10.1117/12.460757 [31] KIM M, LEE G J, CHOI C, et al. An aquatic-vision-inspired camera based on a monocentric lens and a silicon nanorod photodiode array[J]. Nature Electronics, 2020, 3(9): 546-553. doi: 10.1038/s41928-020-0429-5 [32] POTSAID B, BELLOUARD Y, WEN J T. Adaptive Scanning Optical Microscope (ASOM): a multidisciplinary optical microscope design for large field of view and high resolution imaging[J]. Optics Express, 2005, 13(17): 6504-6518. doi: 10.1364/OPEX.13.006504 [33] LECOQ J, SAVALL J, VUČINIĆ D, et al. Visualizing mammalian brain area interactions by dual-axis two-photon calcium imaging[J]. Nature Neuroscience, 2014, 17(12): 1825-1829. doi: 10.1038/nn.3867 [34] BARSON D, HAMODI A S, SHEN X L, et al. Simultaneous mesoscopic and two-photon imaging of neuronal activity in cortical circuits[J]. Nature Methods, 2020, 17(1): 107-113. doi: 10.1038/s41592-019-0625-2 [35] WU Y C, HAN X F, SU Y J, et al. Multiview confocal super-resolution microscopy[J]. Nature, 2021, 600(7888): 279-284. doi: 10.1038/s41586-021-04110-0 [36] WAGNER M J, KIM T H, KADMON J, et al. Shared cortex-cerebellum dynamics in the execution and learning of a motor task[J]. Cell, 2019, 177(3): 669-682.e24. doi: 10.1016/j.cell.2019.02.019 [37] KOROMPILI G, KANAKARIS G, AMPATIS C, et al. A portable, optical scanning microsystem for large field of view, high resolution imaging of biological specimens[J]. Sensors and Actuators A:Physical, 2018, 279: 367-375. doi: 10.1016/j.sna.2018.06.034 [38] MCCALL B, PIERCE M, GRAVISS E A, et al. . Toward a low-cost compact array microscopy platform for detection of tuberculosis[J]. Tuberculosis, 2011, 91 Suppl 1: S54-S60. [39] ORTH A, CROZIER K. Gigapixel fluorescence microscopy with a water immersion microlens array[J]. Optics Express, 2013, 21(2): 2361-2368. doi: 10.1364/OE.21.002361 [40] ORTH A, TOMASZEWSKI M J, GHOSH R N, et al. Gigapixel multispectral microscopy[J]. Optica, 2015, 2(7): 654-662. doi: 10.1364/OPTICA.2.000654 [41] CUI X Q, LEE L M, HENG X, et al. Lensless high-resolution on-chip optofluidic microscopes for Caenorhabditis elegans and cell imaging[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(31): 10670-10675. doi: 10.1073/pnas.0804612105 [42] LEE L M, CUI X Q, YANG C H. The application of on-chip optofluidic microscopy for imaging Giardia lamblia trophozoites and cysts[J]. Biomedical Microdevices, 2009, 11(5): 951-958. doi: 10.1007/s10544-009-9312-x [43] LEE S A, OU X Z, LEE J E, et al. Chip-scale fluorescence microscope based on a silo-filter complementary metal-oxide semiconductor image sensor[J]. Optics Letters, 2013, 38(11): 1817-1819. doi: 10.1364/OL.38.001817 [44] SASAGAWA K, OHTA Y, KAWAHARA M, et al. Wide field-of-view lensless fluorescence imaging device with hybrid bandpass emission filter[J]. AIP Advances, 2019, 9(3): 035108. doi: 10.1063/1.5083152 [45] GUO CH, ZHANG F L, ZHANG X Q, et al. Lensfree super-resolved imaging based on adaptive Wiener filter and guided phase retrieval algorithm[J]. Journal of Optics, 2020, 22(5): 055703. doi: 10.1088/2040-8986/ab8287 [46] JIANG SH W, BIAN Z CH, ZHU J K, et al. High-throughput and field-portable ptychographic lensless on-chip microscopy based on translated pattern modulation[J]. Proceedings of SPIE, 2020, 11250: 112500E. [47] OZCAN A, MCLEOD E. Lensless imaging and sensing[J]. Annual Review of Biomedical Engineering, 2016, 18: 77-102. doi: 10.1146/annurev-bioeng-092515-010849 [48] HAN CH, PANG SH, BOWER D V, et al. Wide field-of-view on-chip talbot fluorescence microscopy for longitudinal cell culture monitoring from within the incubator[J]. Analytical Chemistry, 2013, 85(4): 2356-2360. doi: 10.1021/ac303356v [49] FARSIU S, ROBINSON M D, ELAD M, et al. Fast and robust multiframe super resolution[J]. IEEE Transactions on Image Processing, 2004, 13(10): 1327-1344. doi: 10.1109/TIP.2004.834669 [50] GREENBAUM A, LUO W, KHADEMHOSSEINIEH B, et al. Increased space-bandwidth product in pixel super-resolved lensfree on-chip microscopy[J]. Scientific Reports, 2013, 3(1): 1717. doi: 10.1038/srep01717 [51] WU X J, SUN J S, ZHANG J L, et al. Wavelength-scanning lensfree on-chip microscopy for wide-field pixel-super-resolved quantitative phase imaging[J]. Optics Letters, 2021, 46(9): 2023-2026. doi: 10.1364/OL.421869 [52] ELAD M, HEL-OR Y. A fast super-resolution reconstruction algorithm for pure translational motion and common space-invariant blur[J]. IEEE Transactions on Image Processing, 2001, 10(8): 1187-1193. doi: 10.1109/83.935034 [53] JIANG SH W, GUO CH F, HU P, et al. High-throughput lensless whole slide imaging via continuous height-varying modulation of a tilted sensor[J]. Optics Letters, 2021, 46(20): 5212-5215. doi: 10.1364/OL.437832 [54] VAN PUTTEN E G, AKBULUT D, BERTOLOTTI J, et al. Scattering lens resolves sub-100 nm structures with visible light[J]. Physical Review Letters, 2011, 106(19): 193905. doi: 10.1103/PhysRevLett.106.193905 [55] CHOI Y, YOON C, KIM M, et al. Optical imaging with the use of a scattering lens[J]. IEEE Journal of Selected Topics in Quantum Electronics, 2014, 20(2): 6800213. [56] PARK J H, PARK C, YU H, et al. Subwavelength light focusing using random nanoparticles[J]. Nature Photonics, 2013, 7(6): 454-458. doi: 10.1038/nphoton.2013.95 [57] LI ZH, TAPHANEL M, LÄNGLE T, et al. Confocal fluorescence microscopy with high-NA diffractive lens arrays[J]. Applied Optics, 2022, 61(3): A37-A42. doi: 10.1364/AO.442084 [58] WANG R K K. Signal degradation by multiple scattering in optical coherence tomography of dense tissue: a Monte Carlo study towards optical clearing of biotissues[J]. Physics in Medicine &Biology, 2002, 47(13): 2281-2299. [59] WANG J, ZHANG Y, XU T H, et al. An innovative transparent cranial window based on skull optical clearing[J]. Laser Physics Letters, 2012, 9(6): 469-473. doi: 10.7452/lapl.201210017 [60] CUNHA R, LAFETA L, FONSECA E A, et al. Multimodal microscopy for characterization of amyloid-β plaques biomarkers in animal model of Alzheimer's disease[J]. Analyst, 2021, 146(10): 2945-2954. [61] JIANG L W, WANG X F, WU Z Y, et al. Label-free imaging of brain and brain tumor specimens with combined two-photon excited fluorescence and second harmonic generation microscopy[J]. Laser Physics Letters, 2017, 14(10): 105401. doi: 10.1088/1612-202X/aa7c9a [62] TARANDA J, TURCAN S. 3D whole-brain imaging approaches to study brain tumors[J]. Cancers, 2021, 13(8): 1897. doi: 10.3390/cancers13081897 [63] CALOVI S, SORIA F N, TØNNESEN J. Super-resolution STED microscopy in live brain tissue[J]. Neurobiology of Disease, 2021, 156: 105420. doi: 10.1016/j.nbd.2021.105420 [64] LI A N, GONG H, ZHANG B, et al. Micro-optical sectioning tomography to obtain a high-resolution atlas of the mouse brain[J]. Science, 2010, 330(6009): 1404-1408. doi: 10.1126/science.1191776 [65] RAGAN T, KADIRI L R, VENKATARAJU K U, et al. Serial two-photon tomography for automated ex vivo mouse brain imaging[J]. Nature Methods, 2012, 9(3): 255-258. doi: 10.1038/nmeth.1854 [66] TSAI P S, FRIEDMAN B, IFARRAGUERRI A I, et al. All-optical histology using ultrashort laser pulses[J]. Neuron, 2003, 39(1): 27-41. doi: 10.1016/S0896-6273(03)00370-2 [67] LIN H H, LAI J S Y, CHIN A L, et al. A map of olfactory representation in the Drosophila mushroom body[J]. Cell, 2007, 128(6): 1205-1217. doi: 10.1016/j.cell.2007.03.006 [68] ZHU D, LARIN K V, LUO Q M, et al. Recent progress in tissue optical clearing[J]. Laser &Photonics Reviews, 2013, 7(5): 732-757. [69] UEDA H R, ERTÜRK A, CHUNG K, et al. Tissue clearing and its applications in neuroscience[J]. Nature Reviews Neuroscience, 2020, 21(2): 61-79. doi: 10.1038/s41583-019-0250-1 [70] HAMA H, KUROKAWA H, KAWANO H, et al. Scale: a chemical approach for fluorescence imaging and reconstruction of transparent mouse brain[J]. Nature Neuroscience, 2011, 14(11): 1481-1488. doi: 10.1038/nn.2928 [71] ERTÜRK A, MAUCH C P, HELLAL F, et al. Three-dimensional imaging of the unsectioned adult spinal cord to assess axon regeneration and glial responses after injury[J]. Nature Medicine, 2012, 18(1): 166-171. doi: 10.1038/nm.2600 [72] ZHU D, WANG J, ZHI ZH W, et al. Imaging dermal blood flow through the intact rat skin with an optical clearing method[J]. Journal of Biomedical Optics, 2010, 15(2): 026008. doi: 10.1117/1.3369739 [73] ZHONG H Q, GUO ZH Y, WEI H J, et al. In vitro study of ultrasound and different-concentration glycerol-induced changes in human skin optical attenuation assessed with optical coherence tomography[J]. Journal of Biomedical Optics, 2010, 15(3): 036012. doi: 10.1117/1.3432750 [74] XIA F, GEVERS M, FOGNINI A, et al. Short-wave infrared confocal fluorescence imaging of deep mouse brain with a superconducting nanowire single-photon detector[J]. ACS Photonics, 2021, 8(9): 2800-2810. doi: 10.1021/acsphotonics.1c01018 [75] RYU J, KANG U, KIM J, et al. Real-time visualization of two-photon fluorescence lifetime imaging microscopy using a wavelength-tunable femtosecond pulsed laser[J]. Biomedical Optics Express, 2018, 9(7): 3449-3463. doi: 10.1364/BOE.9.003449 [76] CHENG H, TONG SH, DENG X Q, et al. Deep-brain 2-photon fluorescence microscopy in vivo excited at the 1700 nm window[J]. Optics Letters, 2019, 44(17): 4432-4435. doi: 10.1364/OL.44.004432 [77] CHENG H, TONG SH, DENG X Q, et al. In vivo deep-brain imaging of microglia enabled by three-photon fluorescence microscopy[J]. Optics Letters, 2020, 45(18): 5271-5274. doi: 10.1364/OL.408329 [78] LIU M X, GU B B, WU W B, et al. Binary organic nanoparticles with bright aggregation-induced emission for three-photon brain vascular imaging[J]. Chemistry of Materials, 2020, 32(15): 6437-6443. doi: 10.1021/acs.chemmater.0c01577 [79] LIU W, ZHANG Y H, QI J, et al. NIR-II excitation and NIR-I emission based two-photon fluorescence lifetime microscopic imaging using aggregation-induced emission dots[J]. Chemical Research in Chinese Universities, 2021, 37(1): 171-176. doi: 10.1007/s40242-021-0405-2 [80] MAYERICH D, ABBOTT L, MCCORMICK B. Knife-edge scanning microscopy for imaging and reconstruction of three-dimensional anatomical structures of the mouse brain[J]. Journal of Microscopy, 2008, 231(1): 134-143. doi: 10.1111/j.1365-2818.2008.02024.x [81] SANCATALDO G, GAVRYUSEV V, DE VITO G, et al. Flexible multi-beam light-sheet fluorescence microscope for live imaging without striping artifacts[J]. Frontiers in Neuroanatomy, 2019, 13: 7. doi: 10.3389/fnana.2019.00007 [82] WANG F F, WAN H, MA ZH R, et al. Light-sheet microscopy in the near-infrared II window[J]. Nature Methods, 2019, 16(6): 545-552. doi: 10.1038/s41592-019-0398-7 [83] GELMAN H, GRUEBELE M. Fast protein folding kinetics[J]. Quarterly Reviews of Biophysics, 2014, 47(2): 95-142. doi: 10.1017/S003358351400002X [84] COPOS C, BANNISH B, GASIOR K, et al. . Connecting actin polymer dynamics across multiple scales[M]//SEGAL R, SHTYLLA B, SINDI S. Using Mathematics to Understand Biological Complexity: From Cells to Populations. Cham: Springer, 2021: 7-33. [85] LIU T L, UPADHYAYULA S, MILKIE D E, et al. Observing the cell in its native state: Imaging subcellular dynamics in multicellular organisms[J]. Science, 2018, 360(6386): eaaq1392. doi: 10.1126/science.aaq1392 [86] LI T CH, FU T M, WONG K K L, et al. Cellular bases of olfactory circuit assembly revealed by systematic time-lapse imaging[J]. Cell, 2021, 184(20): 5107-5121.e14. doi: 10.1016/j.cell.2021.08.030 [87] HELL S W, WICHMANN J. Breaking the diffraction resolution limit by stimulated emission: stimulated-emission-depletion fluorescence microscopy[J]. Optics Letters, 1994, 19(11): 780-782. doi: 10.1364/OL.19.000780 [88] RUST M J, BATES M, ZHUANG X W. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM)[J]. Nature Methods, 2006, 3(10): 793-796. doi: 10.1038/nmeth929 [89] BETZIG E, PATTERSON G H, SOUGRAT R, et al. Imaging intracellular fluorescent proteins at nanometer resolution[J]. Science, 2006, 313(5793): 1642-1645. doi: 10.1126/science.1127344 [90] GUSTAFSSON M G L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. Short communication[J]. Journal of Microscopy, 2000, 198(2): 82-87. doi: 10.1046/j.1365-2818.2000.00710.x [91] DIEKMANN R, HELLE Ø I, ØIE C I, et al. Chip-based wide field-of-view nanoscopy[J]. Nature Photonics, 2017, 11(5): 322-328. doi: 10.1038/nphoton.2017.55 [92] ARCHETTI A, GLUSHKOV E, SIEBEN C, et al. Waveguide-PAINT offers an open platform for large field-of-view super-resolution imaging[J]. Nature Communications, 2019, 10(1): 1267. doi: 10.1038/s41467-019-09247-1 [93] HELLE Ø I, COUCHERON D A, TINGUELY J C, et al. Nanoscopy on-a-chip: super-resolution imaging on the millimeter scale[J]. Optics Express, 2019, 27(5): 6700-6710. doi: 10.1364/OE.27.006700 [94] CHEN B CH, LEGANT W R, WANG K, et al. Lattice light-sheet microscopy: imaging molecules to embryos at high spatiotemporal resolution[J]. Science, 2014, 346(6208): 1257998. doi: 10.1126/science.1257998 [95] GAO R X, ASANO S M, UPADHYAYULA S, et al. Cortical column and whole-brain imaging with molecular contrast and nanoscale resolution[J]. Science, 2019, 363(6424): eaau8302. doi: 10.1126/science.aau8302 [96] ZHAO Z Y, XIN B, LI L CH, et al. High-power homogeneous illumination for super-resolution localization microscopy with large field-of-view[J]. Optics Express, 2017, 25(12): 13382-13395. doi: 10.1364/OE.25.013382 [97] MAHECIC D, GAMBAROTTO D, DOUGLASS K M, et al. Homogeneous multifocal excitation for high-throughput super-resolution imaging[J]. Nature Methods, 2020, 17(7): 726-733. doi: 10.1038/s41592-020-0859-z [98] MAU A, FRIEDL K, LETERRIER C, et al. Fast widefield scan provides tunable and uniform illumination optimizing super-resolution microscopy on large fields[J]. Nature Communications, 2021, 12(1): 3077. doi: 10.1038/s41467-021-23405-4 [99] CHMYROV A, LEUTENEGGER M, GROTJOHANN T, et al. Achromatic light patterning and improved image reconstruction for parallelized RESOLFT nanoscopy[J]. Scientific Reports, 2017, 7: 44619. doi: 10.1038/srep44619 [100] CHEN F, TILLBERG P W, BOYDEN E S. Expansion microscopy[J]. Science, 2015, 347(6221): 543-548. doi: 10.1126/science.1260088 [101] TILLBERG P W, CHEN F, PIATKEVICH K D, et al. Protein-retention expansion microscopy of cells and tissues labeled using standard fluorescent proteins and antibodies[J]. Nature Biotechnology, 2016, 34(9): 987-992. doi: 10.1038/nbt.3625 [102] FREIFELD L, ODSTRCIL I, FÖRSTER D, et al. Expansion microscopy of zebrafish for neuroscience and developmental biology studies[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(50): E10799-E10808. [103] GUO F, HOLLA M, DÍAZ M M, et al. A circadian output circuit controls sleep-wake arousal in Drosophila[J]. Neuron, 2018, 100(3): 624-635.e4. doi: 10.1016/j.neuron.2018.09.002 [104] JIN T, GUO H, YAO L, et al. Portable optical-resolution photoacoustic microscopy for volumetric imaging of multiscale organisms[J]. Journal of Biophotonics, 2018, 11(4): e201700250. doi: 10.1002/jbio.201700250 [105] QIN W, JIN T, GUO H, et al. Large-field-of-view optical resolution photoacoustic microscopy[J]. Optics Express, 2018, 26(4): 4271-4278. doi: 10.1364/OE.26.004271 [106] MCNABB R P, POLANS J, KELLER B, et al. Wide-field whole eye OCT system with demonstration of quantitative retinal curvature estimation[J]. Biomedical Optics Express, 2019, 10(1): 338-355. doi: 10.1364/BOE.10.000338 [107] RECHER G, NASSOY P, BADON A. Remote scanning for ultra-large field of view in wide-field microscopy and full-field OCT[J]. Biomedical Optics Express, 2020, 11(5): 2578-2590. doi: 10.1364/BOE.383329 [108] RON A, KALVA S K, PERIYASAMY V, et al. Flash scanning volumetric optoacoustic tomography for high resolution whole-body tracking of nanoagent kinetics and biodistribution[J]. Laser &Photonics Reviews, 2021, 15(3): 2000484. -





 下载:
下载: